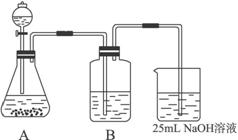
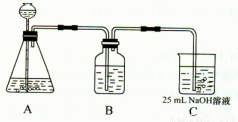
��Ŀ����
Na2CO3��һ�ֺ���Ҫ�Ļ�ѧ���ʣ�ijѧ������ʵ�������Ʊ�Na2CO3�������������Ʊ�ʵ����̣���50 mL��NaOH��Һ����CO2���壬�Ʊ�Na2CO3��Һ��Ϊ�˷�ֹͨ���CO2�������������NaHCO3�������������ʵ�鲽�裺
(��)��25 mL��NaOH��Һ���չ�����CO2���壬��CO2���岻���ܽ⣻
(��)С�������Һ1��2���ӣ�
(��)�ڵõ�����Һ�м�����һ��(25 mL)NaOH��Һ��ʹ��Һ��ֻ�ϣ�
(1)��(��)�У���ͨ��CO2ʱ�����ķ�Ӧ��ѧ����ʽΪ________�������ַ�����Ӧ�����ӷ���ʽΪ________��
��(��)�������Һ��Ŀ����________��
��(��)�л����һ��NaOH��Һ�����ķ�Ӧ�����ӷ���ʽ��________��
(2)����ͬѧ����ƣ���(I)��ʵ��װ�����£�
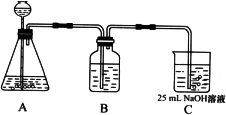
��װ��Aʹ�õ��Լ���________(����)��________��Һ��
��װ��Bʹ�õ��Լ������________(ѡ��ˮ������NaOH��Һ������Na2CO3��Һ������NaHCO3��Һ)��������________���������Bװ�ã����յõ���Na2CO3��Һ�п��ܴ��ڵ�������________��

��12�֣���̼���ƣ�2Na2CO3��3H2O2���׳ƹ���˫��ˮ����һ�ֺܺõ����������������л��������ڣ���������Ӧ����ϴ�ӡ�ӡȾ����֯����ֽ��ҽҩ�����������У������Ʊ�ԭ����·������(ͼ��BC��1��BC��2��Ϊ�ȶ���)��
2Na2CO3 + 3H2O2 �� 2 Na2CO3��3H2O2 ��H<0
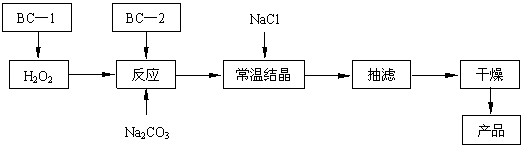 |
�ش��������⣺
�Ź�̼��������������ʱ������������������ (��д���) �������ơ�
A��75%�ƾ� B������ C�� KMnO4ϡ��Һ D��84 ����Һ(NaClO��Һ)
�ƽᾧ�����м����Ȼ��ƽ���������� ��
�Ǽ���BC��2�ȶ����빤ҵ�����к��е�Fe3+���������ȶ���������Ŀ���� ��
����ʵ��ⶨ��Ӧ�¶ȶԲ����Ӱ�����±���
| T/��C | �������ٷֺ��� | ���� |
| 5��10 | 13.94 | 85.49 |
| 10��15 | 14.02 | 85.78 |
| 15��20 | 15.05 | 88.38 |
| 20��25 | 14.46 | 83.01 |
�����ϱ����ݣ�����Ϊ��Ӧ��ѵ��¶�ѡ��ķ�Χ�� ���������� ��
������������������©��һ����������ò�Ʒ����ƫ�ͣ��ò������������� ��
�ʹ�̼���Ƽ��ֽ⣬�����6.72LO2(���)���̼���� g��