��Ŀ����
��14�֣�������(��Ҫ�ɷ�ΪAl2O3��SiO2��Fe2O3)����ȡ��������ԭ�ϡ���ȡ�������Ĺ����������£�

��1����Һ����Ҫ�ɷ��ǣ�д��ѧʽ�� ������������������������
��2��д����Ӧ II �����ӷ���ʽ��
��3����Ϸ�ӦII���ж�������������� ( H+) ����������ǿ������˳���� ������ĸ��ţ�
A��AlO2�D B��OH�D C��SiO32�D
��4��ȡ��Һ������������������ᣬ���ˣ����ö��Ե缫�������Һ���������������������ȫ���ݳ��������������г������ɣ���������ʧ��������ʧ��ԭ��������ӷ���ʽ��ʾΪ��
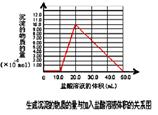
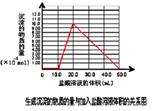
��5��ȡ ��4�� ����Ժ����Һ 10.0 mL��������������Һ��ֻ�������ֵ����ʵ���Ũ�ȵļ������ʣ���������μ���0.100 mol? L��1������Һ��������50.0mL ������Һʱ�����ɵij���ǡ���ܽ⡣
�ټ���50.0mL ������Һ���������ӷ�Ӧ���Ⱥ�˳������Ϊ��
���뻭�����ɳ��������ʵ����������������Ĺ�ϵͼ��


��1����Һ����Ҫ�ɷ��ǣ�д��ѧʽ�� ������������������������
��2��д����Ӧ II �����ӷ���ʽ��
��3����Ϸ�ӦII���ж�������������� ( H+) ����������ǿ������˳���� ������ĸ��ţ�
A��AlO2�D B��OH�D C��SiO32�D
��4��ȡ��Һ������������������ᣬ���ˣ����ö��Ե缫�������Һ���������������������ȫ���ݳ��������������г������ɣ���������ʧ��������ʧ��ԭ��������ӷ���ʽ��ʾΪ��
��5��ȡ ��4�� ����Ժ����Һ 10.0 mL��������������Һ��ֻ�������ֵ����ʵ���Ũ�ȵļ������ʣ���������μ���0.100 mol? L��1������Һ��������50.0mL ������Һʱ�����ɵij���ǡ���ܽ⡣
�ټ���50.0mL ������Һ���������ӷ�Ӧ���Ⱥ�˳������Ϊ��
���뻭�����ɳ��������ʵ����������������Ĺ�ϵͼ��

�š�NaOH��NaAlO2��Na2SiO3
�ơ�CO2+2OH��==CO32��+2H2O CO2��2H2O +2 AlO2��==2Al(OH)3��+HCO3��
�ǡ�b��a��c �ȡ�Al(OH)3 +OH����AlO2��+2H2O
�� �� H����OH��==H2O AlO2-��H��+H2O =Al(OH)3�� Al(OH)3��3H��=Al3����3H2O
��ͼ����������ʾ��
�ơ�CO2+2OH��==CO32��+2H2O CO2��2H2O +2 AlO2��==2Al(OH)3��+HCO3��
�ǡ�b��a��c �ȡ�Al(OH)3 +OH����AlO2��+2H2O
�� �� H����OH��==H2O AlO2-��H��+H2O =Al(OH)3�� Al(OH)3��3H��=Al3����3H2O
��ͼ����������ʾ��

�����������1���������м��������NaOH��Һ�����е�Al2O3��SiO2������Ӧ�õ�NaAlO2��Na2SiO3������NaOH��Һ��������Һ����Ҫ�ɷ���NaAlO2��Na2SiO3��NaOH�������ԵĹ�����Fe2O3����2������NaAlO2��Na2SiO3��NaOH����Һ��ͨ�����CO2��������ӦCO2+2OH��=CO32��+2H2O ��CO2��2H2O +2AlO2��==2Al(OH)3��+HCO3�����õ�Al(OH)3��������3��H2O���������ʣ�Al(OH)3���������ʣ�H2SiO3�����ᡣ������������� ( H+) ����������ǿ������˳����b��a��c����4������NaAlO2��Na2SiO3��NaOH�ļ���������ᷢ����Ӧ�õ�AlCl3��NaCl��H2SiO3����ҺΪAlCl3��NaCl�Ļ����Һ���ö��Ե缫��⣬������������Ӧ��2H++2e-=H2��,�����ƻ��˸�����ˮ�ĵ���ƽ�⣬��Һ��OH-��Ũ������OH-����Һ�е�Al3+������Ӧ�γ�Al(OH)3������������������Ӧ��2Cl-��2e-=Cl2��������Һ�ʼ���ʱ���ַ�����Ӧ��Al(OH)3+ OH-= AlO2-+ 2H2O�����������ܽ����ʧ����5���ټ���50.0mL ������Һ���������ӷ�Ӧ���Ⱥ�˳����H����OH��=H2O ��AlO2-��H��+H2O =Al(OH)3�� Al(OH)3��3H��=Al3����3H2O �� ���ڶ��ߵ����ʵ�����ȣ��������Ƕ�����0.100 mol/L��0.01L=0.001mol��NaOH��HCl��Ӧ����10mlHCl; NaAlO2��HCl��Ӧ�γ�Al(OH)3��������10mlHCl;�ܽ�Al(OH)3��������30mlHCl.ͼ���ͼʾ:

��ϰ��ϵ�д�
 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
�����Ŀ