��Ŀ����
�����15�֣�
��4�������ȿ�����ͬ��ͬ��þ���Ͻ���Ʒ�١��ڡ��ۡ��ܡ��ס��ҡ�������4λͬѧ��ȡ1����Ʒ����������ʵ�飬�ⶨ�Ͻ���þ������������
52����ͬѧȡ��Ʒ��m1 g����������������Һ��Ӧ��Ȼ����ˣ�������Һ��ͨ������Ķ�����̼���壬�����ó������ˡ�ϴ�ӡ���ɡ����գ��õ�����������Ϊm1 g������Ʒ�ٺϽ���þ����������Ϊ_____________��
53����ͬѧȡ��Ʒ��m2 g�����������ᷴӦ��Ȼ��μӹ���������������Һ�����������ˡ�ϴ�ӡ���ɡ����գ��õ�����������Ϊm2 g������Ʒ�ںϽ���þ����������Ϊ____________��
54����ͬѧȡ��Ʒ��m3 g��������ϡ���ᷴӦ�����ֹ�����ȫ�ܽ⣬��״���µõ��������ΪV L������Ʒ����m3��ȡֵ��Χ��___________________________________________��
��ͬѧȡ��ͬ��������Ʒ�ֱܷ��30 mLͬŨ�ȵ����ᷴӦ����ȡ�Ͻ���������������������ת��Ϊ��״�������£�
55��ͨ�����������������ʵ���Ũ�ȡ�
56��ͨ����������Ʒ���кϽ���þ������������
57����c��ʵ������������м���1.0 mol��L-1������������Һ���ٺ�������ʹʣ��Ͻ��е���ǡ����ȫ�ܽ⣿
��4�������ȿ�����ͬ��ͬ��þ���Ͻ���Ʒ�١��ڡ��ۡ��ܡ��ס��ҡ�������4λͬѧ��ȡ1����Ʒ����������ʵ�飬�ⶨ�Ͻ���þ������������
52����ͬѧȡ��Ʒ��m1 g����������������Һ��Ӧ��Ȼ����ˣ�������Һ��ͨ������Ķ�����̼���壬�����ó������ˡ�ϴ�ӡ���ɡ����գ��õ�����������Ϊm1 g������Ʒ�ٺϽ���þ����������Ϊ_____________��
53����ͬѧȡ��Ʒ��m2 g�����������ᷴӦ��Ȼ��μӹ���������������Һ�����������ˡ�ϴ�ӡ���ɡ����գ��õ�����������Ϊm2 g������Ʒ�ںϽ���þ����������Ϊ____________��
54����ͬѧȡ��Ʒ��m3 g��������ϡ���ᷴӦ�����ֹ�����ȫ�ܽ⣬��״���µõ��������ΪV L������Ʒ����m3��ȡֵ��Χ��___________________________________________��
��ͬѧȡ��ͬ��������Ʒ�ֱܷ��30 mLͬŨ�ȵ����ᷴӦ����ȡ�Ͻ���������������������ת��Ϊ��״�������£�
| ʵ����� | a | b | c |
| �Ͻ�����/mg | 510 | 765 | 918 |
| �������/mL | 560 | 672 | 672 |
56��ͨ����������Ʒ���кϽ���þ������������
57����c��ʵ������������м���1.0 mol��L-1������������Һ���ٺ�������ʹʣ��Ͻ��е���ǡ����ȫ�ܽ⣿
52. 47.06%������2�֣�
53. 60%������2�֣�
54. 0.80V��m��1.07V
��2�֣�����д����27V/33.6��m��24V/22.4��9V/11.2��m��3V/2.8�����֣�
55. �������ᷴӦ��ȫ��n(H2)="0.672/22.4" ="0.03" mol����1�֣�
��c(HCl)="0.03��2/0.03=2.0" mol��L-1��1�֣�
56. ��Ͻ���Mg��Al�����ʵ����ֱ�Ϊx��y�����У�
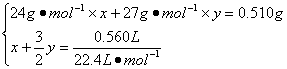
��1�֣�
��ã�x =" y" =" 0.01" mol��1�֣�
��w(Mg)="[(0.01" mol��24 g��mol-1)/0.510 g]��100%=47.06%��1�֣�
57. ����HCl ~ NaCl��Al ~ NaAlO2��֪
n(NaCl)="n(HCl)=2.0" mol��L-1��0.030 L="0.06" mol��1�֣�
n(NaAlO2)="n(Al)=0.01" mol��918/510="0.018" mol��1�֣�
����Na+�غ�ã�n(NaOH)="0.06" mol+0.018 mol="0.078" mol��1�֣�
��V(NaOH)="0.078" mol/1.0 mol��L-1="0.078" L="78" mL ��1�֣�
��c��ʵ��������78 mL������������Һ������ʹʣ��Ͻ��е���ǡ����ȫ�ܽ⡣
���������52.þ���Ͻ��������������Ʒ�Ӧ���ᆳ��������̼��Ӧ���ó������ˡ�ϴ�ӡ���ɡ����գ��õ�����Ϊ����������ӦǰΪþ����Ӧ��Ϊ���������������䣬����Ԫ���غ��֪����������������������Ϊþ������������Ϊ16��3/��16��3+27��2��= 47.06%��53.�÷�Ӧ�õ��Ĺ���Ϊ����þ������Ԫ�������غ㣬����þ��þԪ�ص�������������þ���Ͻ���þԪ�ص�����������Ϊ24/��24+16��= 60%��
54.þ����ϡ���ᷴӦ���ĵ����ͬ������������Ҳ��ͬ���ʲ��ü�ֵ��ȷ���䷶Χ������ȫΪþʱ
��������ΪV /22.4��24=1.07V������ȫΪ��ʱV /22.4��2/3��27=0.80V,��Χ0.80V��m��1.07V��
55.��ͬѧʵ���������������̶���bc�����ᷴӦ��ȫ��a�������Ӧ��ȫ���ʼ��������Ũ�ȸ���bc����������ֱ�ӵó����������ᷴӦ��ȫ��n(H2)="0.672/22.4" ="0.03" mol��
��c(HCl)="0.03��2/0.03=2.0" mol��L-1
56.����a�������з���ʽ�ɽ⣬�ⷨ���𰸣�57.����Ԫ���غ㣬�Ͻ��������ձ�ΪNaAlO2����ʼ������������ձ�Ϊ�Ȼ��ƣ��˿ɳ�Ϊ��̬��������
n(NaCl)="n(HCl)=2.0" mol��L-1��0.030 L="0.06" mol����ԭ���غ㣩
n(NaAlO2)="n(Al)=0.01" mol��918/510="0.018" mol����ԭ���غ㣩
����Na+�غ�ã�n(NaOH)="0.06" mol+0.018 mol="0.078" mol
��V(NaOH)="0.078" mol/1.0 mol��L-1="0.078" L="78" mL ��1�֣�
��c��ʵ��������78 mL������������Һ������ʹʣ��Ͻ��е���ǡ����ȫ�ܽ⡣

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ




