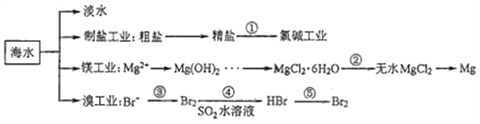
��Ŀ����
����Ŀ��������Ⱦ������������ȫ���ע��
��1������β���д��ڴ�����CO��NO��NO2��̼�⻯����ɲ��ò��ȹ������Ϊ������ʹCO��NOת��Ϊ�������壬��д���仯ѧ����ʽ____________________________
��2�����й������о�������ʯ�������ҵȼúβ���е�����SO2��SO3���͵���NO��NO2�����¹��������ܾ���β�������ܻ��Ӧ�ù㷺��CaSO4��Ca��NO3��2��

��������������γɹ����Ǵ����е�SO2������ˮ����ij���������ڿ����о��������������ᣬ�ù����з�Ӧ�Ļ�ѧ����ʽΪ____________________________�� ______________________________
��CaSO4���Ե���ˮ���Ӳ��ʱ�䡣β����SO2��ʯ���鷴Ӧ����CaSO4�Ļ�ѧ����ʽΪ___________________________
��Ca��NO3��2���Ƴɻ��������������ֽ��������.β����NO��NO2��ʯ���鷴Ӧ����Ca��NO3��2�Ļ�ѧ����ʽ___________________________
��3��������NaClO2��Һ����Һ�ʼ��ԣ���Ϊ���ռ����Ժ���SO2��NOX��ȼú����������������������֪�����������£�ClO2-��ת����ClO2��Cl-��ClO2�ǻ���ɫ��������ˮ�����壬����ǿ�����ԣ�������SO2��NOX���ڹ��ݷ�Ӧ����ͨ�뺬SO2��NO����������Ӧ�¶�Ϊ323K��NaClO2��Һ��Ũ��Ϊ5��10-3mol��L-1����Ӧһ��ʱ�����Һ������Ũ�ȵķ���������±���

��д��NaClO2��Һ������������Ҫ��Ӧ�����ӷ���ʽ_____________________________������ѹǿ��NO��ת����_______�����������������������������������
���������շ�Ӧ�Ľ��У����ռ���Һ��pH��__________���������������������������С������
���𰸡�2CO+2NO![]() 2CO2+N2SO2��H2OH2SO32H2SO3��O2=2H2SO42SO2��O2��2Ca(OH)2=2CaSO4��2H2ONO��NO2��Ca(OH)2=Ca(NO2)2��H2O4NO+3ClO2-+4OH-=4NO3-+3Cl-+2H2O���С
2CO2+N2SO2��H2OH2SO32H2SO3��O2=2H2SO42SO2��O2��2Ca(OH)2=2CaSO4��2H2ONO��NO2��Ca(OH)2=Ca(NO2)2��H2O4NO+3ClO2-+4OH-=4NO3-+3Cl-+2H2O���С
��������
��1��CO��NO�ڴ����������·�Ӧ����CO2��N2���÷�Ӧ�Ļ�ѧ����ʽΪ2CO+2NO![]() 2CO2+N2��
2CO2+N2��
��2���������������γɹ�����SO2����ˮ���������ᣬSO2+H2OH2SO3���ٱ������е��������������ᣬ2H2SO3+O2�T2H2SO4���ʴ�Ϊ��SO2+H2OH2SO3��2H2SO3+O2�T2H2SO4��
��β����SO2��������ʯ���鷴Ӧ����CaSO4��ˮ����Ӧ����ʽΪ��2SO2+O2+2Ca(OH)2�T2CaSO4+2H2O��
��β����NO��NO2��ʯ���鷴Ӧ����Ca(NO2)2��ˮ����Ӧ����ʽΪ��NO+NO2+Ca(OH)2�TCa��NO2��2+H2O��
��3�����������ƾ��������ԣ���NaClO2��Һ�ʼ��ԣ���NaClO2��Һ������������Ҫ��Ӧ�����ӷ���ʽΪ4NO+3ClO2-+4OH-=4NO3-+3Cl-+2H2O������Ӧ�������С�ģ�������ѹǿ��NO��ת������ߣ�
�ڸ��ݷ�Ӧ�ķ���ʽ4NO+3ClO2-+4OH-=4NO3-+3Cl-+2H2O��֪�������շ�Ӧ�Ľ������������ӱ����ģ����ռ���Һ��pH���ͣ��ʴ�Ϊ����С��

����Ŀ����ҵ��ˮ�г�����һ���������Խ�ǿ��Cr2O72�������õζ�ԭ���ⶨCr2O72�������������£�
��������ȡ30.00 mL��ˮ����ƿ�У���������ϡ�����ữ��
����������������ĵ⻯����Һ��ַ�Ӧ��Cr2O72��+6I��+14H+ === 2Cr3++3I2+7H2O��
����������ƿ�е��뼸��ָʾ�����õζ�����ȡ0.1000 molL-1Na2S2O3��Һ���еζ������ݼ�¼���£���I2+2Na2S2O3 === 2NaI+Na2S4O6��
�ζ����� | Na2S2O3��Һ��ʼ����/mL | Na2S2O3��Һ�յ����/mL |
��һ�� | 1.02 | 19.03 |
�ڶ��� | 2.00 | 19.99 |
������ | 0. 20 | a |
��1����������ȡ30.00 mL��ˮѡ���������_____��
��2���������еμӵ�ָʾ��Ϊ_____���ζ��ﵽ�յ��ʵ��������____��
��3����������a �Ķ�����ͼ��ʾ������
�� a=_____��
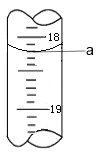
�� Cr2O72���ĺ���Ϊ____gL-1��
��4�����²�������ɷ�ˮ��Cr2O72�������ⶨֵƫ�ߵ���_____��
A. �ζ��յ����ʱ�����ӵζ��ܵĿ̶�
B. ʢװ����Һ����ƿ������ˮϴ����δ�ô���Һ��ϴ
C. �ζ����յ����ʱ���ֵζ��ܼ��촦����һ����Һ
D. ��ȡNa2S2O3��Һ�ĵζ���������ˮϴ��δ�ñ�Һ��ϴ