��Ŀ����
����Ŀ������LiOH ������������Ʊ�����ӵ���������ϡ�LiOH���ɵ�ⷨ�Ʊ������������ͨ���������������
(1) ������ͼװ�õ���Ʊ�LiOH�����缫�����Һ�ֱ�ΪLiOH��LiC1��Һ��
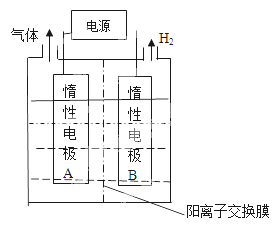
��B�缫�����ҺΪ_______��Һ(�ѧʽ)��
�������缫��ӦʽΪ______________��
����������Li+��_______�缫Ǩ��(����A����B��)��
(2) ��������[��Co(OH)3��Fe(OH)3��]�Ʊ���������Ĺ�����������:
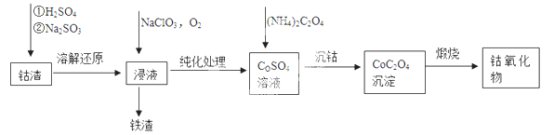
����������Ԫ�صĻ��ϼ�Ϊ________��
��Co (OH) 3�ܽԭ��Ӧ�����ӷ���ʽΪ________��
���ڿ���������CoC2O4�������������CO2����ó�����պ��������Ϊ2.41g��CO2�����Ϊ1.344L(��״��)������������Ļ�ѧʽΪ________��
���𰸡� LiOH 2Cl--2e-=Cl2�� B +3 2Co(OH)2+4H++SO32-=2Co2++SO42-+5H2O Co3O4
����������1���ٵ��ʱB�缫������H2����B�缫��Ϊ��������Һ�е�H+�õ���������H2��OH-Ũ������Li+����B�缫����B�缫��Li+����������B�缫������LiOH����B�缫�����ҺΪLiOH ����B�缫Ϊ��������A�缫Ϊ����������ҺΪLiCl��Cl-ʧ��������Cl2���缫��ӦʽΪ2Cl--2e-=Cl2������Li+�������ƶ���Ҳ����B�缫�ƶ���
��2�������ܽԭ��һ���У�Co(OH)3��Fe(OH)3����Na2SO3��ԭΪCo2+��Fe2+��Ȼ�� Fe2+��NaClO3��O2���������ֱ�����Ϊ+3�۵���Ԫ�أ�Co2+���ᱻ������������������Ԫ�صĻ��ϼ�Ϊ+3����Co(OH)3�����������б�SO32-��ԭ�õ�Co2+�����ӷ���ʽΪ2Co(OH)2+4H++SO32-=2Co2++SO42-+5H2O ����n(CO2)=1.344L��22.4L/mol =0.06mol������CoC2O4�����ʵ���Ϊ0.03mol��Coԭ�ӵĵ����ʵ���Ϊ0.03mol������Ϊ 0.03mol��59g/mol=1.77g����ȼ�պ�Ĺ����к�OԪ�ص�����Ϊ2.41g-1.77g=0.64g������Oԭ�ӵ����ʵ���Ϊ0.64g��16g/mol =0.04mol�������ܵ���������Co��O��ԭ�Ӹ�����Ϊ0.03:0.04=3:4����������Ļ�ѧʽΪCo3O4��

 ����ѧУ�ֲ����ܲ�ϵ�д�
����ѧУ�ֲ����ܲ�ϵ�д� �ƸԺ���ȫ�����Ų��Ծ�ϵ�д�
�ƸԺ���ȫ�����Ų��Ծ�ϵ�д�����Ŀ�������10�֣�
�� 1.0L�����ܱ������з���0.10 mol A (g)����һ���¶Ƚ������·�Ӧ��
A(g) ![]() 2B(g) + C(g) + D(s) ��H =+85��1 kJ / mol
2B(g) + C(g) + D(s) ��H =+85��1 kJ / mol
������������ѹǿ(P)����ʼѹǿP0�ı�ֵ�淴Ӧʱ��(t)�仯���ݼ��±�(��ʾ���ܱ������е�ѹǿ�ȵ����������ʵ���֮�ȣ���
ʱ��t/min | 0 | 1 | 2 | 4 | 8 | 16 | 20 | 25 |
P/P0 | 1.00 | 1.50 | 1.80 | 2.20 | 2.30 | 2.38 | 2.40 | 2.40 |
�ش��������⣺
��1���÷�Ӧ��ƽ�ⳣ������ʽK�� ��
��2��0��2 min�ڵ�ƽ����Ӧ����v(B)= ��
��3����������� A ��ת���ʵ��� ��
A�������¶� B������ϵ��ͨ��A����
C������D�����ʵ��� D������ϵ��ͨ��ϡ������He
��4����ͬ�����£����÷�Ӧ������ʼ��������������ͬ�Ļ�ѧƽ�⣬�� D ��ȡֵ��Χn (D) mol ��
��5����������Ϊ��ѹ�������ı�������ʹ��Ӧ�ﵽ��ͬ���ȣ���ﵽƽ��ʱ B ��Ũ��Ϊ ����������λ��Ч���֣���