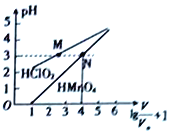
题目内容
【题目】卤族元索的单质和化合物在生产生活中有重要的用途。
(1)基态溴原子的核外电子排布式为[Ar]_________。
(2)在一定浓度的HF溶液中,氟化氢是以缔合形式(HF)2存在的。使氟化氢分子缔合的作用力是_________。
(3)HIO3的酸性_________(填“强于”或“弱于”)HIO4,原因是_________。
(4)ClO2-中心氯原子的杂化类型为_________,ClO3-的空间构型为_________。
(5)晶胞有两个基本要素:①原子坐标参数:表示晶胞内部各微粒的相对位置。下图是CaF2的晶胞,其中原子坐标参数A处为(0,0,0);B处为(![]() ,
, ![]() ,0);C处为(1,1,1)。则D处微粒的坐标参数为_________。
,0);C处为(1,1,1)。则D处微粒的坐标参数为_________。
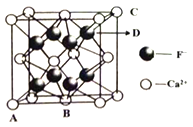
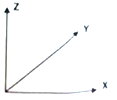
②晶胞参数:描述晶胞的大小和形状。已知CaF2晶体的密度为cg·cm-3,则晶胞中Ca2+与离它最近的F-之间的距离为_________nm(设NA为阿伏加德罗常数的值,用含C、NA的式子表示;相对原子质量:Ca 40 F 19)。
【答案】 3d104s24p5 氢键 弱于 同HIO3相比较,HIO4分子中非羟基氧原子数多,I的正电性高,导致I-O-H中O的电子向I偏移,因而在水分子的作用下,越容易电离出H+,即酸性越强 sp3 三角锥形 (![]() ,
, ![]() ,
, ![]() )
) ![]() ×
×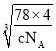 ×107或
×107或![]() ×
×![]() ×107
×107
【解析】(1)基态溴原子的核外电子排布式为[Ar]3d104s24p5。
(2)氟原子的半径小,电负性大,易与氢形成氢键;正确答案:氢键;
(3)同HIO3相比较,HIO4分子中非羟基氧原子数多,I的正电性高,导致I-O-H中O的电子向I偏移,因而在水分子的作用下,越容易电离出H+,即酸性越强;所以HIO3的酸性弱于HIO4;正确答案同上;
(4)ClO2- 为角型,中心氯原子周围有四对价层电子,ClO2-中心氯原子的杂化轨道类型为sp3;根据价层电子对互斥理论,ClO3-中心原子价电子对数为4,采取sp3杂化,轨道呈四面体构型,但由于它配位原子数为3,所以有一个杂化轨道被一个孤电子对占据,所以分子构型为三角锥型。正确答案:sp3 ;三角锥形;
(5)氟化钙晶胞中,阳离子Ca2+呈立方密堆积,阴离子F-填充在四面体空隙中,面心立方点阵对角线的1/4和3/4处;根据晶胞中D点的位置看出,D点的位置均为晶胞中3/4处;正确答案:(![]() ,
, ![]() ,
, ![]() );已知一个氟化钙晶胞中有4个氟化钙;设晶胞中棱长为Lcm;氟化钙的式量为78;根据密度计算公式:
);已知一个氟化钙晶胞中有4个氟化钙;设晶胞中棱长为Lcm;氟化钙的式量为78;根据密度计算公式: ![]() =4×78/NA×L3=C,所以L=
=4×78/NA×L3=C,所以L=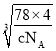 ; 由晶胞中结构看出,与Ca2+最近的F-距离为
; 由晶胞中结构看出,与Ca2+最近的F-距离为![]() L,即
L,即 cm=
cm=![]() ×
× ×107nm;正确答案:
×107nm;正确答案:
(![]() ,
, ![]() ,
, ![]() );
); ×
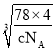 ×107或
×107或![]() ×
×![]() ×107;
×107;

【题目】在一定的温度、压强和钒催化剂存在的条件下,SO2被空气中的O2氧化为SO3。V2O5是钒催化剂的活性成分,郭汗贤等提出:V2O5在对反应I的催化循环过程中,经历了Ⅱ、Ⅲ两个反应阶段,图示如图1:
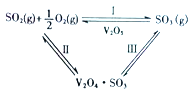
图1
(1)①已知有关气体分子中1mol化学键断裂时需要吸收的能量数据如下:
化学键 | S=O(SO2) | O=O(O2) | S=O(SO3) |
能量/kJ | 535 | 496 | 472 |
由此计算反应Ⅰ的△H=_________kJ·mol-1。
②写出反应Ⅱ的化学方程式_________。
(2)不能说明反应Ⅰ达到平衡状态的是_________。
A.恒容密闭容器中混合气体的压强不再变化
B.恒容密团容器中混合气体的密度不再变化
C.混合气体的总物质的量不再变化
D.混合气体的平均相对分子质量不再变化
E.n(SO2)∶n(O2)∶n(SO3)=2∶1∶2
F.SO2气体的百分含量不再变化
(3)在保持体系总压为105Pa的条件下进行反应SO2+1/2O2![]() SO3,原料气中SO2和O2的物质的量之比m(m=
SO3,原料气中SO2和O2的物质的量之比m(m=![]() )不同时,SO2的平衡转化率与温度(t)的关系如下图所示:
)不同时,SO2的平衡转化率与温度(t)的关系如下图所示:
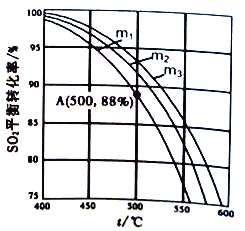
①图中m1、m2、m3的大小顺序为_________,理由是_________。
②反应I的化学平衡常数Kp表达式为_________(用平衡分压代替平衡浓度表示)。图中A点原料气的成分是:n(SO2)=10mol,n(O2)=24.4mol,n(N2)=70mol,达平衡时SO2的分压p(SO2)为_________Pa。(分压=总压×物质的量分数)。
③近年,有人研发出用氧气代替空气的新工艺,使SO2趋于全部转化。此工艺的优点除了能充分利用含硫的原料外,主要还有_________。