��Ŀ����
��14�֣�
�������ڹ�ҵ��ҽҩ���������Ź㷺����;����ͼ��ij��ȤС��ģ����Ʊ��������Ʒ�����Ƶ��������£�
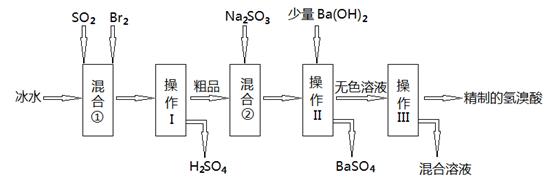
��1����Ϣ�ʹ�ñ�ˮ��Ŀ���� ��
��2������II��III�������� , ��
��3����Ϣ��з�����Ӧ�����ӷ���ʽΪ ��
��4����ҵ����������ʹ���ʯ�Ƶ��廯���к�������Al3+��Fe3+���ʣ������������Լ� ���ѧʽ���������Һ��PHԼΪ8.0���ɳ�ȥ���ʣ�������Һ��PHԼΪ8.0��Ŀ����_______________________________________________________��
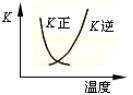
��5��t��ʱ����HBrͨ��AgNO3��Һ�����ɵ�AgBr��ˮ�еij����ܽ�ƽ��������ͼ��ʾ����֪t��ʱAgCl��Ksp=4��l0-10������˵������ȷ���� �� ��
�������ڹ�ҵ��ҽҩ���������Ź㷺����;����ͼ��ij��ȤС��ģ����Ʊ��������Ʒ�����Ƶ��������£�

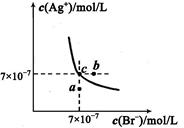
��1����Ϣ�ʹ�ñ�ˮ��Ŀ���� ��
��2������II��III�������� , ��
��3����Ϣ��з�����Ӧ�����ӷ���ʽΪ ��
��4����ҵ����������ʹ���ʯ�Ƶ��廯���к�������Al3+��Fe3+���ʣ������������Լ� ���ѧʽ���������Һ��PHԼΪ8.0���ɳ�ȥ���ʣ�������Һ��PHԼΪ8.0��Ŀ����_______________________________________________________��
��5��t��ʱ����HBrͨ��AgNO3��Һ�����ɵ�AgBr��ˮ�еij����ܽ�ƽ��������ͼ��ʾ����֪t��ʱAgCl��Ksp=4��l0-10������˵������ȷ���� �� ��

| A������Cl-��Br- �Ļ��Һ�еμ���������Һ��һ���Ȳ���AgBr�ij��� |
| B����AgBr������Һ�м���NaBr���壬��ʹ��Һ��c�㵽b�� |
| C��ͼ��a���Ӧ����AgBr�IJ�������Һ |
D����t��ʱ��AgCl(s)+Br-(aq) AgBr(s)+Cl-(aq)ƽ�ⳣ������816 AgBr(s)+Cl-(aq)ƽ�ⳣ������816 |
��ÿ��2�֣�
��1��������ϵ���¶ȣ���ֹ��Ļӷ���2�֣�
��2�����ˣ�����2�֣�
��3�� Br2+SO32-+H2O=2Br-+SO42-+2H+��2�֣�
��4�� CaO��Ca(OH)2��CaCO3��ȷ��Fe3+��Al3+������ȫ�ͷ�ֹ���������ܽ⣨��2�֣�
��5��AB��2�֣�
��1��������ϵ���¶ȣ���ֹ��Ļӷ���2�֣�
��2�����ˣ�����2�֣�
��3�� Br2+SO32-+H2O=2Br-+SO42-+2H+��2�֣�
��4�� CaO��Ca(OH)2��CaCO3��ȷ��Fe3+��Al3+������ȫ�ͷ�ֹ���������ܽ⣨��2�֣�
��5��AB��2�֣�
�����������1��Br2����SO2�ų��ܶ����������ӷ���ʹ�ñ�ˮ��������ϵ�¶ȣ���ֹ��������ʹ��Ӧ��ȫ��
��2���ɹ������̿�֪����������������Һ�壬Ӧ�ǹ��ˣ�������Ϊ���ܵ���Һ��ֵķ��룬Ӧ������
��3����Ϣ��м���Na2SO3��Na2SO3���л�ԭ�ԣ���Br2�������������ӷ���ʽΪ��Br2+SO32-+H2O=2Br-+SO42-+2H+
��4��Ŀ������ȡCaBr2��ͨ��������Һ��PHԼΪ8.0���ɳ�ȥ����Al3+��Fe3+��Ϊ�˷�ֹ�����ʵĽ��룬Ӧ���뺬CaԪ��������H+��Ӧ�����ʣ���CaO��Ca(OH)2��CaCO3��������Һ��PHԼΪ8.0ʱ��Al3+��Fe3+תΪAl(OH)3��Fe(OH)3��������ȥ�����Կ�����Һ��PHԼΪ8.0��Ŀ����ȷ��Fe3+��Al3+������ȫ�ͷ�ֹ���������ܽ⡣
��5��A������ͼ��c����������AgBr��Ksp=7��10-7��7��10-7= 4.9��10-13��С��AgCl��Ksp����ѡ����û�и���Cl?��Br?Ũ�ȣ����Բ�һ���Ȳ���AgBr�ij���������B����AgBr������Һ�м���NaBr���壬�����ܽ�ƽ���ƶ�����Һ��Ϊ������Һ����������c�㵽b�㣬����C��a�����ܽ�ƽ���������£�����a���Ӧ����AgBr�IJ�������Һ����ȷ��D����t��ʱ��AgCl(s)+Br-(aq)
 AgBr(s)+Cl-(aq)ƽ�ⳣ����=c��Cl?��/c(Br?)= c��Cl?��?c(Ag+)/c(Br?) ?c(Ag+)="Ksp(AgCl)/" Ksp(AgBr)= 4��l0-10/4.9��10-13��816����ȷ��
AgBr(s)+Cl-(aq)ƽ�ⳣ����=c��Cl?��/c(Br?)= c��Cl?��?c(Ag+)/c(Br?) ?c(Ag+)="Ksp(AgCl)/" Ksp(AgBr)= 4��l0-10/4.9��10-13��816����ȷ��
��ϰ��ϵ�д�
�����Ŀ
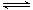 2SO3(g)�������淴Ӧ��ƽ�ⳣ��K���¶ȵı仯������ͼ��ʾ
2SO3(g)�������淴Ӧ��ƽ�ⳣ��K���¶ȵı仯������ͼ��ʾ Ca2+(aq)+20Hһ(aq)������˵����ȷ����( )
Ca2+(aq)+20Hһ(aq)������˵����ȷ����( )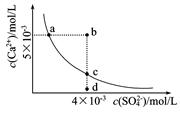
 Ag+(aq)+Cl-(aq)
Ag+(aq)+Cl-(aq) 2Na++S2-
2Na++S2-