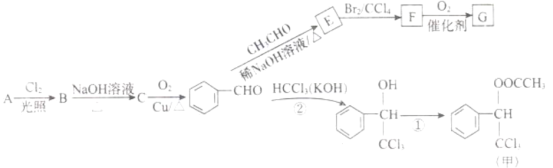
��Ŀ����
����Ŀ���п�Ժһ�����³ɹ�ʵ���˼����Ч������ϩ�������ڴ�����������,�������о����ɻ�ż����Ӧ������ϩ���䷴Ӧ���£� 2CH4(g) ![]() C2H4(g) +2H2(g) ��H>0
C2H4(g) +2H2(g) ��H>0
��ѧ�� | H��H | C��H | C = C | C��C |
E(kJ / mol) | a | b | c | d |
(1)��֪��ػ�ѧ���ļ������ϱ��������Ʊ���ϩ��Ӧ����H=___________ (�ú�a.b.c.d�Ĵ���ʽ��ʾ)��
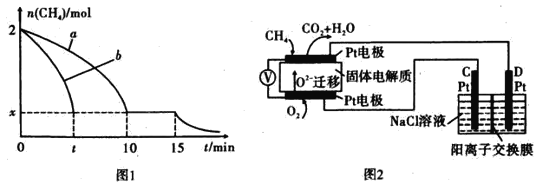
(2)T1�¶�ʱ����1 L�ĺ��ݷ�Ӧ���г���2 molCH4 ,������������Ӧ����Ӧ������ 0~15 min CH4�����ʵ�����ʱ��仯��ͼ1��ʾ�����10-15 minʱH2��Ũ��Ϊ1.6 mol/L��
��0~ 10 min��CH4��ʾ�ķ�Ӧ����Ϊ____mol/(Lmin) o
����ͼ������a������b�ֱ��ʾ���¶�T1ʱ��ʹ��������ͬ���������ͬ�Ĵ���ʱ���ﵽƽ�������n (CH4)�仯���ߣ����б�ʾ����������ϴ�������� ________ (��"a"�� ��b��)��
��15 minʱ,���ı���練Ӧ����,����n( CH4)����ͼ����ʾ�仯����ı������������_____(�δ�һ������)��
(3)ʵ����v��=k��c2(CH4),v��=k��c(C2H4).c2(H2) ����K����K��Ϊ���ʳ��������¶��йأ�T1�¶�ʱk����K���ı�ֵΪ______ (����ֵ)�������¶���T1���ߵ�T2,��Ӧ��������ı���V�� ____V��(����>������������<��)���жϵ�������__________
(4)������Ա����˼���ȼ�ϵ�ز����ڵ�⡣��ͼ2��ʾ��������Dz����� Y2O3�� ZrO2�Ĺ��壬���ڸ����´���O2-
��C����PtΪ_______ ��(ѡ���������������� )��
�ڸõ�ع���ʱ������Ӧ����ʽΪ_____________________ ��
���øõ�ص�ⱥ��ʳ��ˮ��һ��ʱ����ռ�����������������Ϊ112 mL,��������������Һc(OH��)=_______ (������ǰ����Һ�������Ϊ500 mL)��
���𰸡�+ (4b-c-2a) kJmoL-1 0.16 b �����¶� ���Сѹǿ���Сijһ������Ũ�� 12.8 �� �÷�Ӧ��H��0���¶����ߣ�ƽ��������Ӧ�����ƶ�����V�� > V����Ҳ����k������ı�������k�� �� CH4��8e��+4O2��=CO2+2H2O 0.01mol��L��1
��������
�Ÿ����ʱ乫ʽ���м��㡣
����֪������Ũ�ȣ������������������ʣ������������ʣ�����ͼ����������Խ��Ч��Խ�ã���������ڼ��٣�˵��ƽ�������ƶ�������ͨ����Сѹǿ�������¶ȵ���Ӱ��ƽ���ƶ���
��ͨ��ƽ����������ȼ��㣬�����¶ȣ�ƽ�������ƶ�������Ӧ���ʱ��淴Ӧ�������ӵÿ졣
��ԭ�����ȼ�����������������������������������ǵ��ص����������ݵ������д�����缫��Ӧ���ٸ��ݵ�ⷽ��ʽ����������Ũ�ȡ�
���ʱ���ڷ�Ӧ������ܺͼ�ȥ����������ܺͣ���H=(8b ��4b ��c��2a) kJmoL-1 = + (4b��c��2a) kJmoL-1���ʴ�Ϊ��+ (4b��c��2a) kJmoL-1��
(2)��0~ 10 min�������ķ�Ӧ����Ϊ![]() ����������ʵ������������ʣ����CH4������0.16molL-1min-1���ʴ�Ϊ��0.16molL-1min-1��
����������ʵ������������ʣ����CH4������0.16molL-1min-1���ʴ�Ϊ��0.16molL-1min-1��
����ͼ������a������b�ֱ��ʾ���¶�T1ʱ��ʹ��������ͬ���������ͬ�Ĵ���ʱ���ﵽƽ�������n (CH4)�仯���ߣ����б�ʾ����������ϴ����Ч�����ã���Ӧ���ʸ��죬��˴���������ϴ��������b���ʴ�Ϊ��b��
������ͼ��ó���������ʵ�����С��˵�������ƶ���������������¶Ȼ��Сѹǿ���Сijһ������Ũ�ȣ��ʴ�Ϊ�������¶� ���Сѹǿ���Сijһ������Ũ�ȡ�
(3)ʵ����v��=k��c2(CH4)��v��=k��c(C2H4).c2(H2)��T1�¶�ʱv��= v������ʱ����Ũ��Ϊ1.6 mol/L�������Ũ�ȸı���Ϊ1.6 mol/L����ϩ�ı���Ϊ0.8 mol/L�������ƽ��ʱŨ��Ϊ0.4 mol/L����ϩƽ��ʱŨ��Ϊ0.8 mol/L��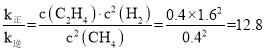 ��k����K���ı�ֵΪ12.8�������¶���T1���ߵ�T2���÷�Ӧ��H��0���¶����ߣ�ƽ��������Ӧ�����ƶ���V��>V������Ӧ��������ı���V�� >V�����жϵ������Ǹ÷�Ӧ��H��0���¶����ߣ�ƽ��������Ӧ�����ƶ�����V�� > V����Ҳ����k������ı�������k�����ʴ�Ϊ�������÷�Ӧ��H��0���¶����ߣ�ƽ��������Ӧ�����ƶ�����V�� > V����Ҳ����k������ı�������k����
��k����K���ı�ֵΪ12.8�������¶���T1���ߵ�T2���÷�Ӧ��H��0���¶����ߣ�ƽ��������Ӧ�����ƶ���V��>V������Ӧ��������ı���V�� >V�����жϵ������Ǹ÷�Ӧ��H��0���¶����ߣ�ƽ��������Ӧ�����ƶ�����V�� > V����Ҳ����k������ı�������k�����ʴ�Ϊ�������÷�Ӧ��H��0���¶����ߣ�ƽ��������Ӧ�����ƶ�����V�� > V����Ҳ����k������ı�������k����
(4)�����ݷ���ԭ�����ȼ�ϼ���������������������������������C����PtΪ�������ʴ�Ϊ������
��ԭ��ع���ʱ����Ϊ���飬�䷴Ӧ����ʽΪCH4��8e��+4O2��= CO2+2H2O���ʴ�Ϊ��CH4��8e��+4O2��= CO2+2H2O��
����ⱥ��ʳ��ˮ�������������������ʵ�����ȣ���ⷴӦ����ʽΪ2NaCl + 2H2O = 2NaOH + H2 ��+ Cl2����һ��ʱ����ռ�����������������Ϊ112 mL��0.005 mol���壬���ݷ���ʽ��ϵ��������������Һn(OH��)=0.005mol��![]() ���ʴ�Ϊ��0.01mol��L��1��
���ʴ�Ϊ��0.01mol��L��1��

����Ŀ��ijͬѧ����֪���ʵ���Ũ�ȵ�NaOH�ⶨδ֪���ʵ���Ũ�ȵ����ᣬ��20.00 ![]() �������������ƿ��,���μ�2-3�η�̪��ָʾ��,��NaOH����Һ���еζ����ظ������ζ�����2-3��,��¼�������¡�
�������������ƿ��,���μ�2-3�η�̪��ָʾ��,��NaOH����Һ���еζ����ظ������ζ�����2-3��,��¼�������¡�
ʵ���� |
| �ζ����ʱ, | ������������/ |
1 | 0.10 | 22.62 | 20.00 |
2 | 0.10 | 22.72 | 20.00 |
3 | 0.10 | 22.80 | 20.00 |
�� �ζ��ﵽ�յ�ı�־��____________________________��
�� ������������,�ɼ�����������Ũ��ԼΪ_______(������λ��Ч����)��
�� �ų���ʽ�ζ��������ݵķ���Ӧ������ͼ��ʾ�����е�________,Ȼ�����ἷѹ������ʹ���첿�ֳ�����Һ��

�� ������ʵ����,���в���(����������ȷ)����ɲⶨ���ƫ�ߵ���________(����ĸ���)��
A. �ζ��յ����ʱ����
B. ��ʽ�ζ���ʹ��ǰ,ˮϴ��δ�ô���������ϴ
C. ��ƿˮϴ��δ����
D. ������![]() �������
�������![]() ����
����
E. ��ʽ�ζ��ܼ��첿��������,�ζ�����ʧ