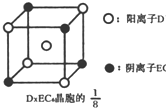
��Ŀ����
����ʵ�����вⶨ����ͭ����ᾧˮ������ʵ�飬��д���пհף�(1)������������ѡ����������(�ñ����ĸ��д)____________________��
A.������ƽ(������) B.�в� C.�Թܼ� D.�ƾ��� E.������ F.������ G.���� H.������ I.ʯ���� J.���ż�
�����������⣬����Ҫ��������_____________________________��
(2)����ʵ��������Ҫ����ƽ�Ͻ����Ĵγ�������һ����_____________�ij������ڶ�����__________________�ij�������������___________________�ij��������Ĵ���___________�ij�������һ�����γ�����Ŀ����___________________________���������Ĵγ�����Ŀ����_____________________________��
(3)ijѧ��ʵ���õ��������ݣ�
����ǰ���� | ���Ⱥ����� | |
W1�������� | W3������+���壩 | W3������+��ˮ����ͭ�� |
5.4 g | 7.9 g | 6.8 g |
��д���ᾧˮ����(x%)�ļ��㹫ʽ(��W1��W2��W3��ʾ)��
x%=______________________��
�����ⶨ�����ƫ����ƫ��?____________��
�����з�����ѡ����ѧ������ʵ������ԭ�������__________(��д��ĸ)��
A.����ǰ����ʱ����δ��ȫ����
B.������μ��Ⱥ��������ϴ�(����0.1 g)
C.���Ⱥ�����δ�������������ȴ
D.���ȹ��������������彦ʧ
(4)���������ۣ�
�ټ�������ͭ����ʱӦע��ʲô��____________________________________________��
��Ҫ��ⶨ���ȷ����������ظ������ı�Ҫ��________________________________��
��1��A��B��D��F��G��H��J ����ǯ�������ǡ�ҩ��
��2����������� ʢ������ͭ��������� ��ȴ�������ͭ������ ����ȴ�������ͭ������ ����������ͭ��������� �������ᾧˮ������
��3��![]() ��100% ƫ�� AD
��100% ƫ�� AD
��4����Ӧ�þƾ��ƻ������ȣ�ͬʱ�ò�����������裬�Է�ֹ����ͭ����ֲ����ȶ��罦 ��Ϊ�����ʵ���ȷ�ȣ����б�Ҫ�����ظ�����
���������ڣ�3������ʵ��IJ��輰ԭ����֪��Ʒ�����нᾧˮ������ΪW2-W3������Ʒ������ӦΪW2-W1�����Խᾧˮ�����ļ���ʽӦΪ��x%=![]() ��100%��
��100%��
��ʵ���������ݴ�����ʽ�ã�x%=![]() ��100%��44%
��100%��44%
��CuSO4��5H2O�����нᾧˮ������ȷֵ�ǣ�
![]() ��100%��36%��44%
��100%��36%��44%
��ʵ��ֵƫ�ߡ�
������ǰ����ʱ����δ��ȫ������������е�ˮ�ֻᱻ����ᾧˮ�У����½ᾧˮ����ƫ�ߣ�����B����������ʵ������Ӱ�죻C���������ʹ�ȵ�CuSO4�������տ����е�ˮ�ֶ�ʹW3��ʵ��ֵƫ�Ӷ�����W2-W3ƫС��ʹx%ҲƫС��D����ȹ����н�ʧ��CuSO4�ᵼ��W3ƫС��ʹx%����

 ʵ������Ũ������ͭ�ķ�Ӧ��ȡ����NaHSO3��ʵ��װ����ͼ��ʾ��
ʵ������Ũ������ͭ�ķ�Ӧ��ȡ����NaHSO3��ʵ��װ����ͼ��ʾ����ش�
��1��ʵ����ȡһ����CuƬ��һ����ŨH2SO4����Բ����ƿ�й��ȣ�����Ӧ����������ƿ�л�������Cuʣ�࣬��H2SO4���Ƿ�ʣ��
��2����Ӧ�����Һ�м���������CuO�����˺���Һ����Ũ������ȴ�ᾧ�Ƶ�����ͭ���壨CuSO4?XH2O��ijС��ͬѧ���ü��ȷ��ⶨ�þ�����ᾧˮX��ֵ��
����ȴ�ᾧ��Ҫ��ýϴ���������ͭ�����ȡ�IJ�����
�������ǵ�ÿһ��ʵ����������ٳ���
�ۼ��ȷ��ⶨ�þ�����ᾧˮX��ֵ���ᾧˮ��ȫʧȥ���жϷ�����
������������ʵ�����ݵ�ƽ��ֵ
| �������� | �����뾧�������� | ���Ⱥ���������������� |
| 11.7g | 22.7g | 18.6g |
��3��װ���ҵ������ǣ�
��4������˵����ȷ���ǣ�
a����װ��ʹ�õIJ��������У��ƾ��ơ������ܡ�����©����Բ����ƿ
b��KMnO4��Һ����β������
c������Ʒ����Һ���뵽��ƿ�У���Ʒ�첻��ɫ��˵����NaHSO3����
d������Ʒ����Һ���뵽��ƿ�У���Ʒ����ɫ��˵��NaOH����ȫת��ΪNaHSO3
e������Ʒ����Һ�������Ը��������Һ�������뵽��ƿ�У������Ϻ�ɫ��˵��NaOH����ȫת��ΪNaHSO3��
 ʵ������Ũ������ͭ�ķ�Ӧ��ȡ���� NaHSO3��ʵ��װ����ͼ��ʾ����ش�
ʵ������Ũ������ͭ�ķ�Ӧ��ȡ���� NaHSO3��ʵ��װ����ͼ��ʾ����ش� ʾ��λ�ڸ�������Ķ�������ģ��û�����Ļ�ѧʽ��
ʾ��λ�ڸ�������Ķ�������ģ��û�����Ļ�ѧʽ��