��Ŀ����
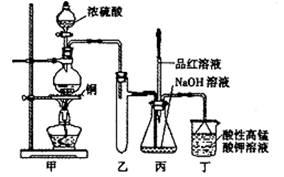
 ʵ������Ũ������ͭ�ķ�Ӧ��ȡ����NaHSO3��ʵ��װ����ͼ��ʾ��
ʵ������Ũ������ͭ�ķ�Ӧ��ȡ����NaHSO3��ʵ��װ����ͼ��ʾ����ش�
��1��ʵ����ȡһ����CuƬ��һ����ŨH2SO4����Բ����ƿ�й��ȣ�����Ӧ����������ƿ�л�������Cuʣ�࣬��H2SO4���Ƿ�ʣ��
��2����Ӧ�����Һ�м���������CuO�����˺���Һ����Ũ������ȴ�ᾧ�Ƶ�����ͭ���壨CuSO4?XH2O��ijС��ͬѧ���ü��ȷ��ⶨ�þ�����ᾧˮX��ֵ��
����ȴ�ᾧ��Ҫ��ýϴ���������ͭ�����ȡ�IJ�����
�������ǵ�ÿһ��ʵ����������ٳ���
�ۼ��ȷ��ⶨ�þ�����ᾧˮX��ֵ���ᾧˮ��ȫʧȥ���жϷ�����
������������ʵ�����ݵ�ƽ��ֵ
| �������� | �����뾧�������� | ���Ⱥ���������������� |
| 11.7g | 22.7g | 18.6g |
��3��װ���ҵ������ǣ�
��4������˵����ȷ���ǣ�
a����װ��ʹ�õIJ��������У��ƾ��ơ������ܡ�����©����Բ����ƿ
b��KMnO4��Һ����β������
c������Ʒ����Һ���뵽��ƿ�У���Ʒ�첻��ɫ��˵����NaHSO3����
d������Ʒ����Һ���뵽��ƿ�У���Ʒ����ɫ��˵��NaOH����ȫת��ΪNaHSO3
e������Ʒ����Һ�������Ը��������Һ�������뵽��ƿ�У������Ϻ�ɫ��˵��NaOH����ȫת��ΪNaHSO3��
��2���ٵõ��ϴ�������ķ����ǣ���ȴ�ᾧ����ˡ�ϴ�ӡ����
��ʵ����Ϊȷ�ⶨ�ᾧˮ��ֵ��Ӧ�ֱ�ⶨ�����������������뾧������������Դ˲ⶨ�����������Ȼ��������Ⱥ������������������֮���ټ���һ�����жϾ����Ƿ���ȫ�ֽ⣬�ܹ�����4�Σ�
�ۼ��������أ����γ�������������0.1g��
�ܸ��ݱ������ݼ�������ͭ�ͽᾧˮ�����ʵ�������������xֵ��
��3����������������������Һ��Ӧ�������գ����װ������Ϊ��ȫƿ��ֹ���������ã�
��4��a��װ�����Ƿ�Һ©����
b�����������Һ�������ն�������
c����Һ���������SO2��Ʒ����ɫ������SO2����ɫ����������˵����NaHSO3��
d����Һ���������SO2��Ʒ����ɫ������SO2����ɫ����������˵����NaHSO3��
e���������ơ����������ơ����������Ժ�����ط�Ӧ��
�ʴ�Ϊ��ʣ�ࣻϡH2SO4��Cu����Ӧ�����ŷ�Ӧ���У�H2SO4Խ��Խϡ������H2SO4һ����ʣ�ࣻ
��2�����ٵõ��ϴ�������ķ����ǣ���ȴ�ᾧ����ˡ�ϴ�ӡ����
�ʴ�Ϊ�����ˡ�ϴ�ӡ����
��ʵ����Ϊȷ�ⶨ�ᾧˮ��ֵ��Ӧ�ֱ�ⶨ�����������������뾧������������Դ˲ⶨ�����������Ȼ��������Ⱥ������������������֮���ټ���һ�����жϾ����Ƿ���ȫ�ֽ⣬�ܹ�����4�Σ�
�ʴ�Ϊ��4��
������ʵ��������������ʼ���ʧˮ������������γ��������������0.1g��
�ʴ�Ϊ�����γ���������0.1g��
�ܸ�ʵ�龧�������Ϊ22.7g-11.7g=11g������ͭ������Ϊ18.6g-11.7g=6.9g��ˮ������Ϊ22.7g-18.6g=4.1g����n��CuSO4��=
| 6.9g |
| 160g/mol |
n��H2O��=
| 4.1g |
| 18g/mol |
| 0.23 |
| 0.043 |
�ʴ�Ϊ��5.28��
��3����������������������Һ��Ӧ�����������գ����װ������Ϊ��ȫƿ��ֹ���������ã�
�ʴ�Ϊ����ֹ����Һ�嵹������У�
��4��a����װ��ʹ�õIJ��������У��ƾ��ơ������ܡ���Һ©����Բ����ƿ����a����
b��KMnO4��Һ����β��������������������Ⱦ�����岻���ŷŵ������У����������Һ�������ն�������b��ȷ��
c������Ʒ����Һ���뵽��ƿ�У���Һ���������SO2��Ʒ����ɫ������SO2����ɫ����������˵����NaHSO��3����c����
d������Ʒ����Һ���뵽��ƿ�У���Ʒ����ɫ��˵����Һ�к��ж����������Ķ����������������ȫ����Ӧ��˵��NaOH����ȫת��ΪNaHSO3����d��ȷ��
e������Ʒ����Һ�������Ը��������Һ�������뵽��ƿ�У������Ϻ�ɫ��������ط�����Ӧ�������������ơ����������ơ����������Ժ�����ط�Ӧ������˵��NaOH����ȫת��ΪNaHSO3����e����
�ʴ�Ϊ��bd��

 ��У����ϵ�д�
��У����ϵ�д���14�֣�ʵ������Ũ������ͭ�ķ�Ӧ��ȡ����NaHSO3��ʵ��װ������ͼ��ʾ��
��ش�
��1��ʵ����ȡһ����CuƬ��һ����ŨH2SO4����Բ����ƿ�й��ȣ�����Ӧ����������ƿ�л�������Cuʣ�࣬������Ϊ����һ������H2SO4ʣ�࣬ԭ����
���ڲ�����ŨH2SO4��ǰ���£���ʹʣ��ͭƬ�ܽ���ټ��� ����д�������ڲ�ͬ�������ʣ���
��2����Ӧ�����Һ�м���������CuO��ʹʣ���H2SO4ȫ��ת��ΪCuSO4�����˺���Һ����Ũ������ȴ�ᾧ�Ƶ�����ͭ���壨CuSO4��XH2O��ijС��ͬѧ���ü��ȷ��ⶨ�þ�����ᾧˮX��ֵ��
�������ǵ�ʵ����������ٳ��� �Ρ�
������������һ��ʵ�������
| �������� | �����뾧�������� | ���Ⱥ���������������� |
| 11.7g | 22.7g | 18.6g |
�����ϱ����ݼ����ж�x��ʵ��ֵ������ֵ��x=5�� ���ƫ��ƫС������
��3��װ���ҵ������ǣ� ��
��4������˵����ȷ���ǣ� ������ţ���
a����װ��ʹ�õIJ��������У��ƾ��ơ������ܡ�����©����Բ����ƿ
b��KMnO4��Һ����β������
c������Ʒ����Һ���뵽��ƿ�У���Ʒ�첻��ɫ��˵����NaHSO3����
d������Ʒ����Һ���뵽��ƿ�У���Ʒ����ɫ��˵��NaOH����ȫת��ΪNaHSO3
e������Ʒ����Һ�������Ը��������Һ�������뵽��ƿ�У������Ϻ�ɫ��˵��NaOH����ȫת��ΪNaHSO3
f����װ�û�������ҩƷ������ȡ���ռ���������������
 ʵ������Ũ������ͭ�ķ�Ӧ��ȡ���� NaHSO3��ʵ��װ����ͼ��ʾ����ش�
ʵ������Ũ������ͭ�ķ�Ӧ��ȡ���� NaHSO3��ʵ��װ����ͼ��ʾ����ش� ʵ������Ũ������ͭ�ķ�Ӧ��ȡ����NaHSO3��ʵ��װ����ͼ��ʾ��
ʵ������Ũ������ͭ�ķ�Ӧ��ȡ����NaHSO3��ʵ��װ����ͼ��ʾ��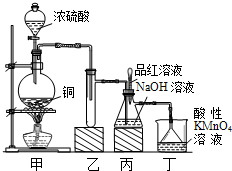 ʵ������Ũ������ͭ�ķ�Ӧ��ȡ����NaHSO3��ʵ��װ����ͼ��ʾ��
ʵ������Ũ������ͭ�ķ�Ӧ��ȡ����NaHSO3��ʵ��װ����ͼ��ʾ��