��Ŀ����
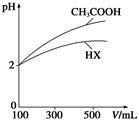
�����£���ijһԪ��HA��NaOH��Һ�������ϣ�������Һ��Ũ�Ⱥͻ�Ϻ�������Һ��pH���±���
��ش��������⣺
(1)�������������ʵ���������Ӽ�����������������a(�����Һ��pH)��˵��HB��ǿ�ỹ������________��
(2)�������������ʵ�����������������������c1�Ƿ�һ������0.2 mol/L________��(��ǡ���)�������Һ������Ũ��c(B��)��c(K��)�Ĵ�С��ϵ��________��
A��ǰ�ߴ� B�����ߴ�
C��������� D�����ж�
(3)�ӱ���ʵ����������HB��________��(�ǿ��������)���û����Һ������Ũ���ɴ�С��˳����________��
(4)����ʵ�����û����Һ����ˮ�������c(OH��)��________mol/L��д���û����Һ��������ʽ�ľ�ȷ���(���������Ƽ���)��c(K��)��c(B��)��_mol/L��
| ʵ���� | HB���ʵ���Ũ��(mol/L) | KOH���ʵ���Ũ��(mol/L) | �����Һ��pH |
| �� | 0.2 | 0.2 | pH��a |
| �� | c1 | 0.2 | pH��7 |
| �� | 0.1 | 0.1 | pH>7 |
| �� | 0.1 | 0.1 | pH��9 |
��ش��������⣺
(1)�������������ʵ���������Ӽ�����������������a(�����Һ��pH)��˵��HB��ǿ�ỹ������________��
(2)�������������ʵ�����������������������c1�Ƿ�һ������0.2 mol/L________��(��ǡ���)�������Һ������Ũ��c(B��)��c(K��)�Ĵ�С��ϵ��________��
A��ǰ�ߴ� B�����ߴ�
C��������� D�����ж�
(3)�ӱ���ʵ����������HB��________��(�ǿ��������)���û����Һ������Ũ���ɴ�С��˳����________��
(4)����ʵ�����û����Һ����ˮ�������c(OH��)��________mol/L��д���û����Һ��������ʽ�ľ�ȷ���(���������Ƽ���)��c(K��)��c(B��)��_mol/L��
(1)��a��7����HBΪǿ�ᣬ��a>7����Ϊ����
(2)��C��(3)����c(Na��)>c(B��)>c(OH��)>c(H��)
(4)10��5��10��5��10��9
(2)��C��(3)����c(Na��)>c(B��)>c(OH��)>c(H��)
(4)10��5��10��5��10��9
(1)��Ϊ��һԪ���һԪ������ʵ���Ũ�ȵ������ϣ�˵��ǡ����ȫ��Ӧ�����ɵIJ�����KB����KB��Һ��������a��7��˵��HB��ǿ�ᣬ��a>7��˵����Һ��B����ˮ����Լ��ԣ���HB�����ᡣ(2)c1��һ������0.2����HB��ǿ��ʱ����0.2����HB������ʱ������0.2����ΪpH��7��˵��c(H��)��c(OH��)����ô���ݵ���غ��֪��һ����c(K��)��c(B��)��(3)�ɱ���ʵ�����ݿ�֪������Һ����������ʵ���Ũ�Ȼ�Ϻ�pH>7��˵��B��ˮ�⣬�õ�HB�����ᣬ��������֪��(4)����ʵ����������Һ��pH��9��˵����Һ��c(H��)��10��9mol/L������ˮ���������c(H��)��c(OH��)�� ��10��5(mol/L)���ɵ���غ�ã�c(K��)��c(H��)��c(OH��)��c(B��)����c(Na��)��c(B��)��c(OH��)��c(H��)��(10��5��10��9)mol/L��
��10��5(mol/L)���ɵ���غ�ã�c(K��)��c(H��)��c(OH��)��c(B��)����c(Na��)��c(B��)��c(OH��)��c(H��)��(10��5��10��9)mol/L��
 ��10��5(mol/L)���ɵ���غ�ã�c(K��)��c(H��)��c(OH��)��c(B��)����c(Na��)��c(B��)��c(OH��)��c(H��)��(10��5��10��9)mol/L��
��10��5(mol/L)���ɵ���غ�ã�c(K��)��c(H��)��c(OH��)��c(B��)����c(Na��)��c(B��)��c(OH��)��c(H��)��(10��5��10��9)mol/L��
��ϰ��ϵ�д�
 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�
�����Ŀ
 H++HS-��HS-
H++HS-��HS- SO32�� + 2HClO
SO32�� + 2HClO