��Ŀ����
�о���ѧ��Ӧԭ���������������Ǻ�������ġ�
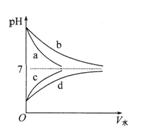
��1�����й��ڴ����������ȷ���� ����д���ţ���
a���������pH�Ĵ����������ȫ��NaOH��Һ�кͣ�����NaOH�����ʵ���һ����
b���������Һ�м���һ����NaOH���壬��Һ�ĵ�������ǿ
c����ˮϡ�ʹ�����Һ����Һ�е���������Ũ�Ⱦ���С
d�������£�������Һ��ˮ�ĵ���̶ȱȴ�ˮ��С
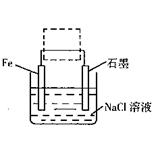
��2���������������洦�ɼ��������ĵ绯��ʴԭ����ͼ��ʾ��
��д��ʯī�缫�ĵ缫��Ӧʽ ��
�ڽ���װ�������ļ��ɳ�Ϊ�����绯ѧ������װ�ã�������ͼ���߿�����ʾλ�������ġ�
��д���ĺ�ʯī�缫�ĵ缫��Ӧʽ ��
��3���ٸ�¯���������лᷢ����Ӧ��FeO��s��+CO��g�� Fe��s��+CO2��g����
Fe��s��+CO2��g����
��֪��Fe��s��+1/2O2��g��=FeO��s����H= -272kJ��mol-1
C��s��+O2��g��=CO2��g�� ��H= -393��5kJ��mol-1
2C��s��+O2��g��=2CO��g�� ��H= -22lkJ��mol-1
��FeO��s��+CO��g�� Fe��s��+CO2��g��
Fe��s��+CO2��g��
��H= ��
��һ���¶��£���ij�ܱ������м�������FeO��
������һ������CO���壬��Ӧ������CO��CO2��
Ũ����ʱ��Ĺ�ϵ��ͼ��ʾ��ӿ�ʼ���ﵽƽ������У�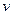 ��CO��= ��
��CO��= ��
��4��������һ�ֺ�ɫ���ϣ���ɷ���Fe2O3����һ��������������160mL 5mol��L-1�����У��ټ���һ��������ǡ����ȫ�ܽ⣬�ռ�������2.24L����״����������⣬��Һ����Fe3+����μӷ�Ӧ�����۵�����Ϊ ��
��1�����й��ڴ����������ȷ���� ����д���ţ���
a���������pH�Ĵ����������ȫ��NaOH��Һ�кͣ�����NaOH�����ʵ���һ����
b���������Һ�м���һ����NaOH���壬��Һ�ĵ�������ǿ
c����ˮϡ�ʹ�����Һ����Һ�е���������Ũ�Ⱦ���С
d�������£�������Һ��ˮ�ĵ���̶ȱȴ�ˮ��С
��2���������������洦�ɼ��������ĵ绯��ʴԭ����ͼ��ʾ��

��д��ʯī�缫�ĵ缫��Ӧʽ ��
�ڽ���װ�������ļ��ɳ�Ϊ�����绯ѧ������װ�ã�������ͼ���߿�����ʾλ�������ġ�
��д���ĺ�ʯī�缫�ĵ缫��Ӧʽ ��
��3���ٸ�¯���������лᷢ����Ӧ��FeO��s��+CO��g��
 Fe��s��+CO2��g����
Fe��s��+CO2��g������֪��Fe��s��+1/2O2��g��=FeO��s����H= -272kJ��mol-1
C��s��+O2��g��=CO2��g�� ��H= -393��5kJ��mol-1
2C��s��+O2��g��=2CO��g�� ��H= -22lkJ��mol-1
��FeO��s��+CO��g��
 Fe��s��+CO2��g��
Fe��s��+CO2��g����H= ��
��һ���¶��£���ij�ܱ������м�������FeO��
������һ������CO���壬��Ӧ������CO��CO2��
Ũ����ʱ��Ĺ�ϵ��ͼ��ʾ��ӿ�ʼ���ﵽƽ������У�
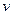 ��CO��= ��
��CO��= ��
��4��������һ�ֺ�ɫ���ϣ���ɷ���Fe2O3����һ��������������160mL 5mol��L-1�����У��ټ���һ��������ǡ����ȫ�ܽ⣬�ռ�������2.24L����״����������⣬��Һ����Fe3+����μӷ�Ӧ�����۵�����Ϊ ��
��1��bd (2) ��O2+2H2O+4e-=4OH- ���� ��2Cl-��2e-=Cl2
(3) �� -11Kj/mol ��0.0625mol/(Lmin) (4)11.2g
(3) �� -11Kj/mol ��0.0625mol/(Lmin) (4)11.2g
��

��ϰ��ϵ�д�
�����Ŀ