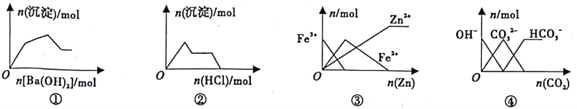
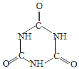
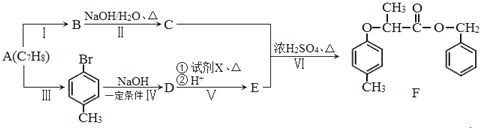
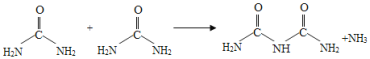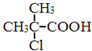��Ŀ����
����Ŀ����һƿ�������Һ��ֻ���ܺ��� NH4+��Na+��Mg2+��Ba2+��Fe3+��Cl����Br����I����CO32����SO42���еļ��֣���Ũ�Ⱦ�Ϊ 0.1mol L��1����������ʵ�飺
��ȡ������Һ���μ���������Һ�����ԣ�����������
��ȡ������Һ���μ�����������ˮ���ټӵ�����Һ����Һ����
��ȡ������Һ����������μ��� NaOH ��Һ�����ԣ������о�����������������Һ��Ϊ���ȷݣ���һ�ݼ��ȣ�������ų����ڶ�����Һ�м���Na2CO3 ��Һ���а�ɫ�������ɡ����н��۲���ȷ����
A.�϶����е��������� NH4+��Ba2+
B.�϶����е���������I����Cl����Br��
C.�϶������е������� Fe3+��CO32����SO42��
D.����ȷ���Ƿ��е������� Na+
���𰸡�D
��������
��ȡ������Һ���μ���������Һ�����ԣ�����������˵������Һ�в�����![]() ��
��
��ȡ������Һ���μ�����������ˮ���ټӵ�����Һ����Һ������˵��ԭ��Һ�к���I-����Fe3+��I-���ܹ��棬�ʲ���Fe3+��
��ȡ������Һ����������μ��� NaOH ��Һ�����ԣ������о�����������˵��ԭ��Һ�в�����Mg2+��������Һ��Ϊ���ȷݣ���һ�ݼ��ȣ�������ų���˵������Һ�к���NH3H2O��˵��ԭ��Һ�к���![]() ���ڶ�����Һ�м���Na2CO3 ��Һ���а�ɫ�������ɣ�˵��ԭ��Һ�к���Ba2+����Ba2+��
���ڶ�����Һ�м���Na2CO3 ��Һ���а�ɫ�������ɣ�˵��ԭ��Һ�к���Ba2+����Ba2+��![]() ���ܴ������棬��ԭ��Һ�в�����
���ܴ������棬��ԭ��Һ�в�����![]() ��
��
ͨ��ʵ��ȷ���������У�Ba2+��![]() ��I-����������Ũ�Ⱦ�Ϊ 0.1mol L-1��ϵ���غ��֪��������һ������Cl-��Br-�������Һ��һ��������Na+��
��I-����������Ũ�Ⱦ�Ϊ 0.1mol L-1��ϵ���غ��֪��������һ������Cl-��Br-�������Һ��һ��������Na+��
��������������D��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�