��Ŀ����
8�������жϲ���ȷ���ǣ�������| A�� | ��AgCl�ij����ܽ�ƽ����ϵ�У���������ˮ��Ksp��AgCl������ | |
| B�� | ��0.1mol•L-1Na2CO3��Һ�м�������NaOH���壬c��CO32-����c��Na+�������� | |
| C�� | NaOH��CH3COONa�Ļ����Һ�У�c��Na+��+c��H+��=c��OH-��+c��CH3COO-�� | |
| D�� | �����£�$\frac{{K}_{W}}{c{��H}^{+}��}$=0.1mol•L-1 ����Һ�У�Na+��K+��CO32-��NO3-�����ӿɴ������� |
���� A���������ܽ�ƽ�ⳣ��ֻ���¶�Ӱ�죻
B����̼������Һ�м���NaOH��Ӱ��̼������ӵ�ˮ��ƽ�⣻
C���ݵ���غ������
D�������£�$\frac{{K}_{W}}{c{��H}^{+}��}$=0.1mol•L-1 ����Һ�У�c��H+��=10-13mol/L����ҺpH=13��
��� �⣺A����AgCl�ij����ܽ�ƽ����ϵ�У���������ˮ��Ksp��AgCl�����䣬��A����
B����0.1mol•L-1Na2CO3��Һ�м�������NaOH���壬����̼������ӵ�ˮ�⣬c��CO32-������c��Na+������B��ȷ��
C��NaOH��CH3COONa�Ļ����Һ�У�ֻ��4�����ӣ��ݵ���غ����c��Na+��+c��H+��=c��OH-��+c��CH3COO-������C��ȷ��
D��pH=13����Һ�У�Na+��K+��CO32-��NO3-�����ܴ������棬��D��ȷ��
��ѡA��
���� ���⿼���˳������ܽ�ƽ�ⳣ��������ƽ����ƶ�����Һ�еĵ���غ㡢���ӹ��棬ע�����֪ʶ�Ļ��ۣ���Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
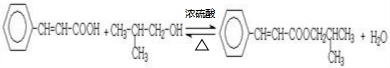
5��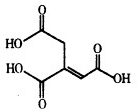 ��ͷ��Ľṹ��ʽ��ͼ��ʾ�����й�����ͷ���˵����ȷ���ǣ�������
��ͷ��Ľṹ��ʽ��ͼ��ʾ�����й�����ͷ���˵����ȷ���ǣ�������
 ��ͷ��Ľṹ��ʽ��ͼ��ʾ�����й�����ͷ���˵����ȷ���ǣ�������
��ͷ��Ľṹ��ʽ��ͼ��ʾ�����й�����ͷ���˵����ȷ���ǣ�������| A�� | ��ͷ���������ͬϵ�� | |
| B�� | ��ͷ����̼Ԫ�ص���������Ϊ41.4% | |
| C�� | ��ͷ���ܷ����кͷ�Ӧ��ˮ�ⷴӦ��������Ӧ | |
| D�� | ��1mol��ͷ�����Һ��������3mol NaOH��3mol Br2 |
13���й����з�Ӧ��˵���У���ȷ���ǣ�������
| A�� | п��Ͷ��Cu��NO3��2��Һ�У���Ӧ������������� | |
| B�� | 22.4L Cl2ͨ������NaOH��Һ����Ӧʱת�Ƶĵ�����Ϊ2NA | |
| C�� | lmol Na�ڿ����м���ȼ�գ���Ӧʱת�Ƶĵ�����ΪNA | |
| D�� | �����ʵ�����MgCl2��Ba��OH��2�� HCl��Һ��ϣ�Mg2++2OH-=Mg��OH��2�� |
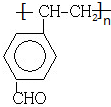
 �����̬ԭ����26��������ͬ�ĵ��ӣ�
�����̬ԭ����26��������ͬ�ĵ��ӣ�
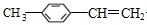
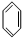 +CH3Br$\stackrel{����}{��}$
+CH3Br$\stackrel{����}{��}$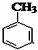 +HBr
+HBr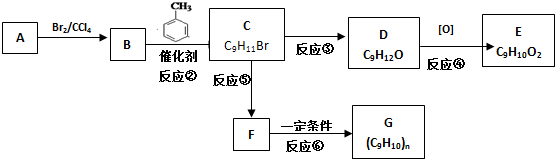
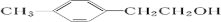 ��
�� $\stackrel{һ������}{��}$
$\stackrel{һ������}{��}$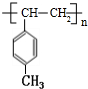
 +
+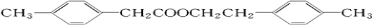
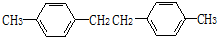 ��
��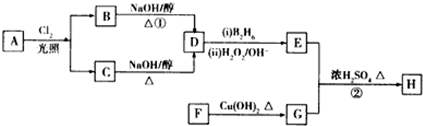
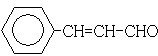 ��
�� ��
�� ��
��