��Ŀ����
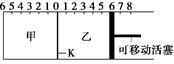
��ͼ������K�������ƶ������г���2 mol A��1 mol B�����г���2 mol C��1 mol He����ʱKͣ��0����������Ӧ2A(g)��B(g)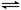 2C(g)���ﵽƽ��ָ����¶ȡ������й�˵������ȷ����
2C(g)���ﵽƽ��ָ����¶ȡ������й�˵������ȷ����
 2C(g)���ﵽƽ��ָ����¶ȡ������й�˵������ȷ����
2C(g)���ﵽƽ��ָ����¶ȡ������й�˵������ȷ����
| A����ƽ�����K����ͣ�������̶�0��2֮�� |
| B����ƽ��ʱKͣ�������1���������ͣ�����Ҳ�6�� |
| C���ﵽƽ��ʱ����������B�����ʵ���С������������B�����ʵ��� |
| D�����ݸ���K���������ж��������ߵķ�Ӧ�Ƿ�ﵽƽ�� |
B
��Ӧ�������С�Ŀ��淴Ӧ�������ڷ�Ӧ������������������ʵ������ӣ�������������ʵ������٣����K�����ƶ��������ǿ��淴Ӧ�����Լ�����������ʵ���һ������2mol����˸���K����ͣ�������̶�0��2֮�䣬A��ȷ���������൱���ڼ������Ļ����ϣ�ƽ��ʱͨ��ϡ�����壬��ѹǿ���������£�ƽ��������A��B�ķ����ƶ�������ѡ��C��ȷ����K���ٻ���ʱ��˵����Ӧ�ﵽƽ��״̬��D��ȷ����ƽ��ʱKͣ�������1������������������ʵ����仯������1����λ������B����ȷ����ѡB��

��ϰ��ϵ�д�
 ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�
�����Ŀ
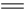 C(g) +D(g) ��H-T��S="(-4500+11T)" J/mol��Ҫ��ֹ��Ӧ�������¶ȱ��룺
C(g) +D(g) ��H-T��S="(-4500+11T)" J/mol��Ҫ��ֹ��Ӧ�������¶ȱ��룺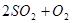
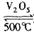
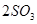 �������Ӧ���ܱ������н��У�����˵���������
�������Ӧ���ܱ������н��У�����˵���������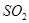 �����ʵ������ٸı�ʱ���÷�Ӧ�ﵽ��ƽ��״̬
�����ʵ������ٸı�ʱ���÷�Ӧ�ﵽ��ƽ��״̬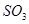 ��Ũ��һ�����
��Ũ��һ�����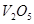 ������Ϊ�˼ӿ췴Ӧ���ʣ��������Ч��
������Ϊ�˼ӿ췴Ӧ���ʣ��������Ч�� H2��g����CO��g����
H2��g����CO��g����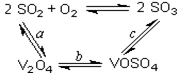
 SO3+ V2O4
SO3+ V2O4 
 2SO2+O2 ��550 ��ʱ��ƽ�ⳣ��K= ��
2SO2+O2 ��550 ��ʱ��ƽ�ⳣ��K= ��
 2C(g) ��H��0
2C(g) ��H��0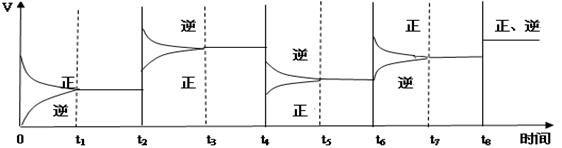
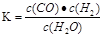
 CO(g)��Cl2(g)����H<0 �����й�˵����ȷ����(����)
CO(g)��Cl2(g)����H<0 �����й�˵����ȷ����(����)