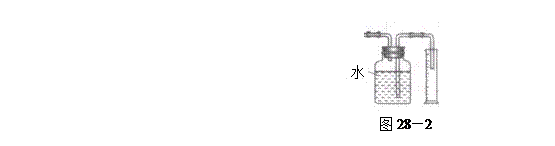��Ŀ����
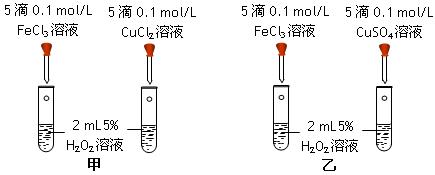
��14�֣�����ͭ��һ��Ӧ�ü���㷺�Ļ���ԭ�ϡ��Ʊ�����ͭ������ѧʵ���ѧ��һ����Ҫ��ʵ�顣����ͭ������ϡ����ֱ�ӷ�Ӧ��ʵ���н�Ũ����ִμ��뵽ͭ����ϡ�����У�����ʹ֮��Ӧ��ȫ��ͨ���������ᾧ�õ�����ͭ���壨װ����ͼ1��2��ʾ����
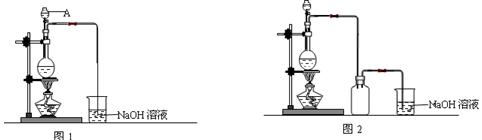
��1��ͼ1�У�A����������Ϊ ����ƿ�з��������ӷ�Ӧ����ʽΪ ��
��2��ͼ2��ͼ1�ĸĽ�װ�ã��Ľ���Ŀ���� ��
��3��Ϊ������ɫ��ѧ��Ҫ��ij�о���ѧϰС�����������ƣ�
��һ�飺����Ϊ��������
����1���Կ���Ϊ����������ͭ��������B�з������գ�ʹͭ�������ַ�Ӧ��������ͭ���ٽ�����ͭ��ϡ���ᷴӦ��
����2��������������ֱ��ͨ�뵽ͭ����ϡ����Ļ�����У������ڳ����¼�������Ӧ����ӦҺ�м�FeSO4��Fe2��SO4��3����Ӧ��ȫ�������м����ʼ���pH��3��4������Fe��OH��3���������ˡ��������ᾧ������������ѭ��ʹ�á�����֪Fe��OH��3��Cu��OH��2��ȫ����ʱ��pH�ֱ�Ϊ3.7��6.4����
��ش��������⣺
�ٷ���1�е�B���������� ��
�ڷ���2�м������� ������ĸ��ţ���a��CaO b��CuCO3 c��CaCO3
�ڶ��飺��������Ϊ��������
��3.2gͭ˿�ŵ�45 mL 1.5mol/L��ϡ�����У�������50�档����18mL 10%��H2O2����Ӧ0.5h�����µ�60�棬������Ӧ1 h���ˡ������ᾧ����ѹ���˵ȣ�������95%�ľƾ���ϴ�����ɣ���CuSO4��5H2O 10.6g����ش��������⣺
�۸÷�Ӧ�����ӷ�Ӧ����ʽΪ ��
�ܿ����¶���50���60����ȵ�ԭ��Ϊ ��������þƾ���ϴ���ŵ��� ��
�����������������У���������ɫ��ѧ������� �����һ�顱�ڶ��顱���������� ��

��1��ͼ1�У�A����������Ϊ ����ƿ�з��������ӷ�Ӧ����ʽΪ ��
��2��ͼ2��ͼ1�ĸĽ�װ�ã��Ľ���Ŀ���� ��
��3��Ϊ������ɫ��ѧ��Ҫ��ij�о���ѧϰС�����������ƣ�
��һ�飺����Ϊ��������
����1���Կ���Ϊ����������ͭ��������B�з������գ�ʹͭ�������ַ�Ӧ��������ͭ���ٽ�����ͭ��ϡ���ᷴӦ��
����2��������������ֱ��ͨ�뵽ͭ����ϡ����Ļ�����У������ڳ����¼�������Ӧ����ӦҺ�м�FeSO4��Fe2��SO4��3����Ӧ��ȫ�������м����ʼ���pH��3��4������Fe��OH��3���������ˡ��������ᾧ������������ѭ��ʹ�á�����֪Fe��OH��3��Cu��OH��2��ȫ����ʱ��pH�ֱ�Ϊ3.7��6.4����
��ش��������⣺
�ٷ���1�е�B���������� ��
�ڷ���2�м������� ������ĸ��ţ���a��CaO b��CuCO3 c��CaCO3
�ڶ��飺��������Ϊ��������
��3.2gͭ˿�ŵ�45 mL 1.5mol/L��ϡ�����У�������50�档����18mL 10%��H2O2����Ӧ0.5h�����µ�60�棬������Ӧ1 h���ˡ������ᾧ����ѹ���˵ȣ�������95%�ľƾ���ϴ�����ɣ���CuSO4��5H2O 10.6g����ش��������⣺
�۸÷�Ӧ�����ӷ�Ӧ����ʽΪ ��
�ܿ����¶���50���60����ȵ�ԭ��Ϊ ��������þƾ���ϴ���ŵ��� ��
�����������������У���������ɫ��ѧ������� �����һ�顱�ڶ��顱���������� ��
����14�֣�
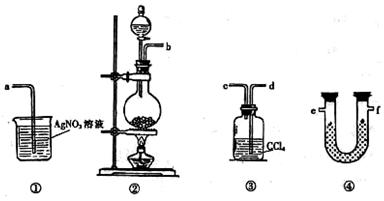
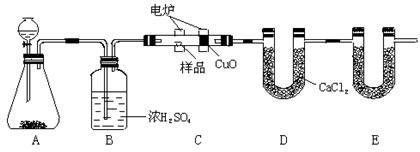
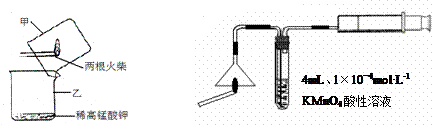
��1����Һ©����1�֣���Cu+4H++2NO3��=Cu2++2NO2��+2H2O(��NO�ķ���ʽҲ��)��2�֣���
��2����ȫƿ����ֹ�����ȣ���1�֣�
��3����������1�֣� ��b��1�֣� ��Cu+H2O2+2H+=Cu2++2H2O��2�֣�
�ܷ�ֹ˫��ˮ�ֽ⣨1�֣����ƾ���ˮ�����Ҽ��ӷ������پ����ܽ⣨1�֣����ڶ���(2��)
���ɣ���һ���еķ���1��Ҫ���ȣ�������Դ������2���ò�Ʒ������Ԫ�����ʡ����ڶ��鷽�������������к����壬�����ò�Ʒ���Ƚϸߡ���2�֣�
��1����Һ©����1�֣���Cu+4H++2NO3��=Cu2++2NO2��+2H2O(��NO�ķ���ʽҲ��)��2�֣���
��2����ȫƿ����ֹ�����ȣ���1�֣�
��3����������1�֣� ��b��1�֣� ��Cu+H2O2+2H+=Cu2++2H2O��2�֣�
�ܷ�ֹ˫��ˮ�ֽ⣨1�֣����ƾ���ˮ�����Ҽ��ӷ������پ����ܽ⣨1�֣����ڶ���(2��)
���ɣ���һ���еķ���1��Ҫ���ȣ�������Դ������2���ò�Ʒ������Ԫ�����ʡ����ڶ��鷽�������������к����壬�����ò�Ʒ���Ƚϸߡ���2�֣�
��

��ϰ��ϵ�д�
 ��ְٷְټ���ϵ�д�
��ְٷְټ���ϵ�д�
�����Ŀ



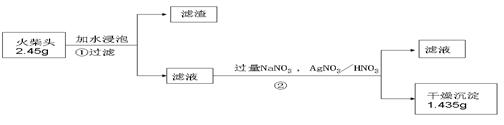
 �ڻ���Ƥ�ܾ�����ȥ����ɸ�ʵ�飬����������Ϊ�����ң�����ȷ������˳���ǣ�
�ڻ���Ƥ�ܾ�����ȥ����ɸ�ʵ�飬����������Ϊ�����ң�����ȷ������˳���ǣ�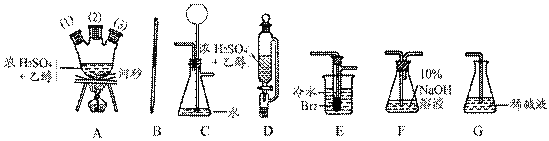
 ____________________��
____________________�� �飬��Ӧ��������ʵ��ǰ����������ɴ˵õ���ϩ������
�飬��Ӧ��������ʵ��ǰ����������ɴ˵õ���ϩ������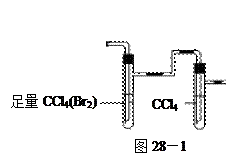 ����II����E��������װ�ò��������ͼ28��2��ʾװ�ý���ʵ�飬��Ӧ���������ϩ��������ɴ������ϩ������
����II����E��������װ�ò��������ͼ28��2��ʾװ�ý���ʵ�飬��Ӧ���������ϩ��������ɴ������ϩ������