��Ŀ����
6��̫���ܵ����ͨ�����ЧӦ���߹⻯ѧЧӦֱ�Ӱѹ���ת���ɵ��ܵ�װ�ã�����ϳ������裬����ͭ�������Ȼ������1���صĻ�̬ԭ�ӵĵ����Ų�ʽ��1s22s22p63s23p63d104s24p1����[Ar]3d104s24p1����
��2����Ϊ��4����Ԫ�أ����ڵ�Ԫ��������壬��3��Ԫ�صĵ�һ�����ܴӴ�С˳��ΪBr��As��Se����Ԫ�ط��ű�ʾ����
��3����̬SeO3���ӵ����幹��Ϊƽ�������Σ�
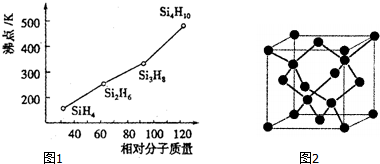
��4�����飨SinH2n+2���ķе�������Է��������ı仯��ϵ��ͼ1��ʾ���������ֱ仯��ϵ��ԭ���ǣ��������Է�������Խ���Ӽ䷶�»���Խǿ��
��5������Ԫ�ش���ͬһ�������Ԫ�ؾ���ȱ�����ԣ��仯�����������мӺ��ԣ�������ᣨH3BO3����ˮ��Һ������ˮ��Ӧ����[B��OH��4]-������һԪ��������ʣ���[B��OH��4]-��B��ԭ���ӻ�����Ϊsp3��
��6������Cu�����백ˮ����������ⶼ���ܷ�Ӧ�������백ˮ��������Ļ����Һ��Ӧ����ͭ�������ӵ���Һ����÷�Ӧ�����ӷ���ʽΪCu+H2O2+4NH3•H2O=Cu��NH3��42++2OH-+4H2O��
��7�����ʯ�ľ�����ͼ2������������Ľṹ����ʯ���ƣ���֪�����߳�Ϊ361.5pm����������������ܶ���$\frac{4��25}{��{361.5��1{0}^{-10}��}^{3}{N}_{A}}$g•cm��3��ֻҪ������ʽ�����ؼ������ֵ������٤��������NA��ʾ����
���� ��1������31��Ԫ�أ�����ԭ�Ӻ�������Ų����ɿ���д�������Ų�ʽ��
��2���顢����������Ԫ�ض��ǵ�4���ڷǽ���Ԫ�أ�ͬһ����Ԫ��������ҵ�һ�����ܳ��������ƣ�����Ԫ��ԭ��4p�ܼ��ǰ����ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�أ��ʵ�һ������Br��As��Se���ݴ˴��⣻
��3����̬SeO3����������ԭ�ӵļ۲���Ӷ��������жϷ��ӹ��ͣ�
��4�����飨SinH2n+2�����Ƿ��Ӿ��壬���Ӿ���ķе�ߵ�ȡ���ڷ��Ӽ��������������Ӽ�����������Է��������Ĵ�С�йأ��ݴ˴��⣻
��5�����ݼ۲���ӶԻ�������ȷ�����ӻ���ʽ��
��6������������ԭ��Ӧ��Ԫ�غ͵���غ㣬��д�����ӷ���ʽ��
��7�����þ�̯�����㾧������ԭ�Ӻ͵�ԭ�ӵ���Ŀ�����ݦ�=$\frac{m}{V}$���㵪������ܶȣ�
��� �⣺��1������31��Ԫ�أ�����ԭ�Ӻ�������Ų����ɿ���д�������Ų�ʽΪ��1s22s22p63s23p63d104s24p1����[Ar]3d104s24p1����
�ʴ�Ϊ��1s22s22p63s23p63d104s24p1����[Ar]3d104s24p1����
��2���顢����������Ԫ�ض��ǵ�4���ڷǽ���Ԫ�أ�ͬһ����Ԫ��������ҵ�һ�����ܳ��������ƣ�����Ԫ��ԭ��4p�ܼ��ǰ����ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�أ��ʵ�һ������Br��As��Se��
�ʴ�Ϊ��Br��As��Se��
��3����̬SeO3����������ԭ�ӵļ۲���Ӷ���Ϊ$\frac{6+0}{2}$=3���µ��Ӷԣ����Է��ӹ���Ϊƽ�������Σ�
�ʴ�Ϊ��ƽ�������Σ�
��4�����飨SinH2n+2�����Ƿ��Ӿ��壬���Ӿ���ķе�ߵ�ȡ���ڷ��Ӽ��������������Ӽ�����������Է��������Ĵ�С�йأ��������Է�������Խ���Ӽ䷶�»���Խǿ��
�ʴ�Ϊ���������Է�������Խ���Ӽ䷶�»���Խǿ��
��5��[B��OH��4]-��B�ļ۲���Ӷ�=4+$\frac{1}{2}$��3+1-4��1��=4�����Բ�ȡsp3�ӻ���
�ʴ�Ϊ��sp3��
��6������Cu�����백ˮ����������ⶼ���ܷ�Ӧ�������백ˮ��������Ļ����Һ��Ӧ��˵�������ܻ���ٽ������������ʹ�ͬ���õĽ�������й�������Ϊ������������Cu2+�γ������ӣ�������ٽ�ʹ��Ӧ���У�����ʽ�ɱ�ʾΪ��Cu+H2O2+4NH3•H2O=Cu��NH3��42++2OH-+4H2O��
�ʴ�Ϊ��Cu+H2O2+4NH3•H2O=Cu��NH3��42++2OH-+4H2O��
��7�����þ�̯����Ͻ��ʯ�ľ�����֪���ڽ��ʯ�ľ����к��е�̼ԭ����Ϊ4+8��$\frac{1}{8}$+6��$\frac{1}{2}$=8�����Ե���������ԭ�Ӻ͵�ԭ�ӵ���Ŀ����4���������߳�Ϊ361.5pm�����ݦ�=$\frac{m}{V}$��֪��������ܶ�Ϊ$\frac{\frac{4��25}{{N}_{A}}}{��{361.5��1{0}^{-10}��}^{3}}$g•cm��3=$\frac{4��25}{��{361.5��1{0}^{-10}��}^{3}{N}_{A}}$g•cm��3��
�ʴ�Ϊ��$\frac{4��25}{��{361.5��1{0}^{-10}��}^{3}{N}_{A}}$��
���� ������Ҫ�����˺�������Ų�����һ�����ܡ����ӿռ乹�͡��ӻ���ʽ�������ܶȵļ��㣬�Ѷ��еȣ�����ʱҪע��Ի���֪ʶ��������ã�

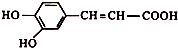 ���йؿ������˵���в���ȷ���ǣ�������
���йؿ������˵���в���ȷ���ǣ�������| A�� | ���������ʽ��C9H8O4 | |
| B�� | ��������Է�����ԭ��ȡ�����Ӿ۵ȷ�Ӧ | |
| C�� | 1mol�����������4molH2�����ӳɷ�Ӧ | |
| D�� | 1mol���������������3molNaHCO3 |
| A�� | ͨ��������һ��Ϊ��ص����� | |
| B�� | �����������1��molˮʱ��ת��4��mol���� | |
| C�� | ������ӦΪ4OH--4e-=2H2O+O2�� | |
| D�� | �ŵ�ʱCO32-���ƶ� |
| A�� | ������ˮ�У�Cl-��NO3-��Na+��SO32- | |
| B�� | c��H+��=1.0��10-13mol/L��Һ�У�C6H5O-��K+��SO42-��Br- | |
| C�� | ���ȳʻ�ɫ����Һ�У�I-��Cl-��NO3-��Na+ | |
| D�� | ������Al3+����Һ�У�K+��Na+��NO3-��ClO- |
| Ԫ�� | �����Ϣ |
| X | X�Ļ�̬ԭ�Ӻ���3���ܼ����е��ӣ���ÿ���ܼ��ϵĵ�������� |
| Y | ���³�ѹ�£�Y�����ǵ���ɫ���壬���ڻ�ɽ�ڸ������� |
| Z | Z��Yͬ���ڣ�Z�ĵ縺�Դ���Y |
| W | W��һ�ֺ��ص�������Ϊ63��������Ϊ34 |
��2��XY2��һ�ֳ��õ��ܼ���XY2�ķ����д���2���Ҽ�����H-Y��H-Z���ֹ��ۼ��У����ļ��Խ�ǿ����H-Cl�������ϳ�����H-S��
��3��XZ4������X��sp3��ʽ�ӻ���
��4��д��WԪ�ص�ԭ�Ӻ�������Ų�ʽ1s22s22p63s23p63d104s1��
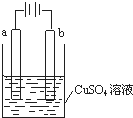
| A�� | ��a��bΪʯī��ͨ���a�缫�Ϸ����ķ�ӦΪCu2++2e-�TCu | |
| B�� | ��a��bΪͭ��ͨ���a�缫��������� | |
| C�� | ��aΪͭ��bΪ����ͨ���Cu2+��a�缫�ƶ� | |
| D�� | ��aΪ��ͭ��bΪ��ͭ��ͨ���b�缫�������� |
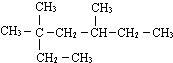 3��3��5-��������
3��3��5-��������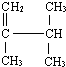 2��3-����-1-��ϩ
2��3-����-1-��ϩ 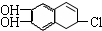 �к��еĹ����ŵ�����Ϊ�ǻ���̼̼˫������ԭ�ӣ�
�к��еĹ����ŵ�����Ϊ�ǻ���̼̼˫������ԭ�ӣ�