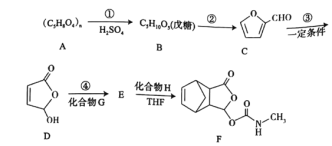
题目内容
【题目】氨是最重要的氮肥,是产量最大的化工产品之一。其合成原理为:N2(g)+3H2(g)![]() 2NH3(g)ΔH=-92.4 kJ·mol-1,在密闭容器中,投入1mol N2和3 mol H2在催化剂作用下发生反应:
2NH3(g)ΔH=-92.4 kJ·mol-1,在密闭容器中,投入1mol N2和3 mol H2在催化剂作用下发生反应:
(1)测得反应放出的热量_________92.4kJ。(填“小于”,“大于”或“等于”)
(2)当反应达到平衡时,N2和H2的浓度比是___________;N2和H2的转化率比是___________。
(3)升高平衡体系的温度(保持体积不变),混合气体的平均相对分子质量________。(填“变大”、“变小”或“不变”)
(4)当达到平衡时,充入氩气,并保持压强不变,平衡将___________(填“正向”、“逆向”或“不”)移动。
(5)若容器恒容、绝热,加热使容器内温度迅速升至原来的2倍,平衡将____________(填“向左移动”、“向右移动”或“不移动”)。达到新平衡后,容器内温度________(填“大于”、“小于”或“等于”)原来的2倍。
【答案】小于 1:3 1:1 变小 逆向 平衡向左移动 小于
【解析】
(1)热化学方程式为N2(g)+3H2(g)![]() 2NH3(g)ΔH=-92.4 kJ·mol-1表示1mol氮气(g)与3mol 氢气(g)完全反应生成2mol氨气(g)反应的热量为92.4kJ,由于该反应是可逆反应,加入1molN2和3molH2不可能完全反应,所以放出的热量总是小于92.4kJ,
2NH3(g)ΔH=-92.4 kJ·mol-1表示1mol氮气(g)与3mol 氢气(g)完全反应生成2mol氨气(g)反应的热量为92.4kJ,由于该反应是可逆反应,加入1molN2和3molH2不可能完全反应,所以放出的热量总是小于92.4kJ,
故答案为:小于;
(2)对N2(g)+ 3H2(g)![]() 2NH3(g) ΔH<0,在密闭容器中,开始时n(N2):n(H2)=1:3,反应时消耗n(N2):n(H2)=1:3,故平衡时n(N2):n(H2)=1:3,所以c(N2):c(H2)=1:3,转化率之比为1:1。
2NH3(g) ΔH<0,在密闭容器中,开始时n(N2):n(H2)=1:3,反应时消耗n(N2):n(H2)=1:3,故平衡时n(N2):n(H2)=1:3,所以c(N2):c(H2)=1:3,转化率之比为1:1。
答案为:1:3;1:1;
(3)升高温度,平衡向逆反应方向移动,气体的总物质的量增大,总质量不变,故平均相对分子质量变小。
答案为:变小;
(4)达平衡后,保持压强不变,充入氩气,使体系体积增大,浓度减小,相当于减小压强,使平衡逆向移动。
答案为:逆向;
(5)恒容时升高温度至原来的2倍,根据勒夏特列原理,平衡向吸热反应的方向移动,即向左移动,达新平衡后,由于吸热容器内温度小于原来温度的2倍。
答案为:平衡向左移动;小于。

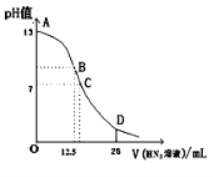
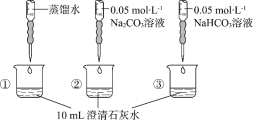
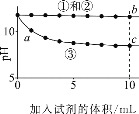
【题目】实验小组利用传感器探究Na2CO3和NaHCO3的性质。
实验操作 | 实验数据 |
测量下述实验过程的pH变化
|
|
下列分析不正确的是
A.①与②的实验数据基本相同,说明②中的OH-未参与该反应
B.加入试剂体积相同时,②所得沉淀质量大于③所得沉淀质量
C.从起始到a点过程中反应的离子方程式为:Ca2++2OH-+2HCO3-=CaCO3↓+2H2O+CO32-
D.b点对应溶液中水的电离程度小于c点对应溶液中水的电离程度