��Ŀ����
17��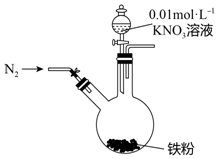 ijͬѧ�����������������ԭNO3-�ѳ�����ˮ�������Ρ���������Ϻ���������װ��̽��������KNO3��Һ�ķ�Ӧ��ʵ�鲽�輰�������£�
ijͬѧ�����������������ԭNO3-�ѳ�����ˮ�������Ρ���������Ϻ���������װ��̽��������KNO3��Һ�ķ�Ӧ��ʵ�鲽�輰�������£�| ʵ�鲽�� | ʵ������ |
| 1�����ɼУ�����ͨ��N2 | |
| 2������pHΪ2.5��0.01mol/L����KNO3��Һ100mL | ���۲����ܽ⣬��Һ��dz��ɫ�� ���۲����ܽ��ʣ�����۱������������ɫ���ʸ��ţ� |
| 3����Ӧֹͣ�ε���������Բ����ƿȡ�� | ��ƿ���������ɫû�з����仯�� |
| 4��ʣ�������� | ����İ�ɫ���ʱ�Ϊ���ɫ�� |
��2����ɫ������Fe��OH��2���û�ѧ����ʽ�������Ϊ���ɫ��ԭ��4Fe��OH��2+O2+2H2O=4Fe��OH��3��
��3��Ϊ��̽���Һ�ijɷ֣���ͬѧ��һ�����������ʵ�飺
| ʵ�鲽�� | ʵ������ |
| 1��ȡ������Һ���Թ��У������м���KSCN��Һ | ��ҺҺ�ޱ仯 |
| 2����������Һ��Ϊ���ݣ�һ���е�����������һ���еμ�ϡ���� | ������Һ����Ϊ��ɫ |
| 3����ȡ������Һ���Թ��У������м���ŨNaOH��Һ�����ȣ����Թܿڷ���ʪ��ĺ�ɫʯ����ֽ�� | ���������ɣ�������ʹ��ɫʯ����ֽ������ |
��ii������2�еμ�ϡ�������Һ����dz��ɫ��ɺ�ɫ���������ӷ���ʽ������ԭ��3Fe2++NO3-+4H+=3Fe3++NO��+2H2O��
���� ��1��������������Ӱ��Fe����������ӵķ�Ӧ��
��2�����۲����ܽ⣬��Һ��Ϊdz��ɫ��˵����Fe2+���ɣ����Լ���Fe2+ˮ��õ�Fe��OH��2������������������Ϊ������������ɫ��Ϊ���ɫ��
��3��ȡ������Һ���Թ��У������м���KSCN��Һ����ҺҺ�ޱ仯˵���������ӣ���������Һ��Ϊ���ݣ�һ���е�����������һ���еμ�ϡ���ᣬ������Һ����Ϊ��ɫ˵����Ӧ�����������ӣ��������������������������������������ӣ������������Һ����������Ӿ���������Ҳ�������������ӣ�֤������������Ӻ��������ӣ���ȡ������Һ���Թ��У������м���ŨNaOH��Һ�����ȣ����Թܿڷ���ʪ��ĺ�ɫʯ����ֽ�����������ɣ�������ʹ��ɫʯ����ֽ������֤��ԭ��Һ�к�笠����ӣ���Һ�д����������ӡ���������ӡ�笠����ӣ��������Ӻ��л�ԭ�ԣ�����������������¾��������ԣ��������ӱ�����Ϊ�����ӣ�
��� �⣺��1���õ����ž�װ���п��������������������Fe��NO3-���ӷ�Ӧ�ĸ��ţ�
�ʴ�Ϊ����ֹ�����е�O2��Fe��NO3-��Ӧ�ĸ��ţ�����Ӱ�췴Ӧ������жϣ�
��2�����۲����ܽ⣬��Һ��Ϊdz��ɫ��˵����Fe2+���ɣ������۲����ܽ⣬���ú��֣�ʣ����������������ɫ����Լ���Fe2+ˮ��õ�Fe��OH��2����ɫ����Ϊ������������������Ϊ������������ɫ��Ϊ���ɫ����Ӧ����ʽΪ��4Fe��OH��2+O2+2H2O=4Fe��OH��3��
�ʴ�Ϊ��Fe��OH��2��4Fe��OH��2+O2+2H2O=4Fe��OH��3��
��3����i��ȡ������Һ���Թ��У������м���KSCN��Һ����ҺҺ�ޱ仯˵���������ӣ���������Һ��Ϊ���ݣ�һ���е�����������һ���еμ�ϡ���ᣬ������Һ����Ϊ��ɫ˵����Ӧ�����������ӣ��������������������������������������ӣ������������Һ����������Ӿ���������Ҳ�������������ӣ�֤������������Ӻ��������ӣ���ȡ������Һ���Թ��У������м���ŨNaOH��Һ�����ȣ����Թܿڷ���ʪ��ĺ�ɫʯ����ֽ�����������ɣ�������ʹ��ɫʯ����ֽ������֤��ԭ��Һ�к�笠����ӣ���Һ��һ������NH4+��Fe2+��NO3-���ӣ�
�ʴ�Ϊ��NH4+��Fe2+��NO3-��
��ii����Һ�д���NH4+��Fe2+��NO3-���ӣ��������ᣬ������Ӧ��3Fe2++NO3-+4H+=3Fe3++NO��+2H2O�����ɵ�Fe3+��SCN-��Ӧʹ��Һ�ʺ�ɫ��
�ʴ�Ϊ��3Fe2++NO3-+4H+=3Fe3++NO��+2H2O��
���� ���⿼��̽��ʵ�鷽�����⣬�Ƕ�ѧ���ۺ������Ŀ��飬��Ҫѧ���߱���ʵ�Ļ�����������������������ע��Ի���֪ʶ���������գ���Ŀ�Ѷ��еȣ�

| ѡ�� | ʵ����� | ʵ������ | ���� |
| A | ��Na2SiO3��Һ�еμ�1�η�̪��Ȼ����μ���ϡ��������ɫ��ȥ | 2min�������� | ���ԣ�HCl��H2SiO3 |
| B | ��SiO2�м�������ˮ���� | SiO2�ܽ� | SiO2��H2SiO3������ |
| C | �����£���Ũ������Ͷ����Ƭ | ��Ƭ���ܽ� | �����£�����Ũ�����Ӧ |
| D | ��ij��Һ���ȵμ�������ˮ��һ�����μ�KSCN��Һ | �μ���ˮʱ���������μ�KSCN��Һ���Һ��ɺ�ɫ | ԭ��Һ�к���Fe2+��û��Fe3+ |
| A�� | A | B�� | B | C�� | C | D�� | D |
| ѡ�� | ʵ����������� | ʵ����� |
| A | ��ij��Һ�м��������ữ���Ȼ�����Һ���а�ɫ�������� | ����Һ��һ������SO42- |
| B | ��ij��Һ�еμ�NaOH��Һ��������ɫ���� | ����Һ��һ������Al3+ |
| C | ��ij��Һ�м���NaOHŨ��Һ�����ȣ�������������ʹʪ��� ��ɫʯ����ֽ���� | ����Һ��һ������NH4+ |
| D | ��ij�Ϻ�ɫ��Һ�м��˹���Na2SO3��Һ����Һ�Ϻ�ɫ��ȥ | ����Һ��һ������MnO4- |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | NaCl | B�� | Cl2 | C�� | HCl | D�� | H2 |
��֪��
 ��a��b��c�������㣩
��a��b��c�������㣩����˵������ȷ���ǣ�������
| A�� | ��Ӧ�������������������������� | |
| B�� | �Ͽ�1molH-H����1molI-I�������������ڶϿ�2molH-I���������� | |
| C�� | �Ͽ�2molH-I����������ԼΪ��c+b+a��kJ | |
| D�� | ���ܱ������м���2molH2��2molI2����ַ�Ӧ�ų���������С2akJ |
| A�� |  ʵ�飺���ã��ϲ���Һ��ɫ���ֲ��� | |
| B�� |  ʵ�飺�Թ���Һ�г������ݣ���Һ�ȳ��ֻ��Ǻ����� | |
| C�� |  ʵ�飺��ϡHNO3Ƭ�̣���Һ�������ݲ��������ƿ��ʼ�ձ�����ɫ | |
| D�� |  ʵ�飺���������Һ�ʺ��ɫ��ֹͣ���ȣ��÷�ɢϵ�ܲ��������ЧӦ |
 ���ڿ���������������ҪӦ�ã�ij��ȤС����0.50mol•L-1KI��0.2%������Һ��0.20mol•L-1K2S2O8��0.10mol•L-1Na2S2O3���Լ���̽����Ӧ�����Ի�ѧ��Ӧ���ʵ�Ӱ�죮
���ڿ���������������ҪӦ�ã�ij��ȤС����0.50mol•L-1KI��0.2%������Һ��0.20mol•L-1K2S2O8��0.10mol•L-1Na2S2O3���Լ���̽����Ӧ�����Ի�ѧ��Ӧ���ʵ�Ӱ�죮��֪��S2O2-8+2I-�T2SO2-4+I2������
I2+2S2O2-3�T2I-+S4O2-6���죩
��1��Ϊ̽����Ӧ��Ũ�ȶԻ�ѧ��Ӧ���ʵ�Ӱ�죬��Ƶ�ʵ�鷽�����±���
| ʵ����� | ���V/mL | ||||
| K2S2O8��Һ | ˮ | KI��Һ | Na2S2O3��Һ | ������Һ | |
| �� | 10.0 | 0.0 | 4.0 | 4.0 | 2.0 |
| �� | 9.0 | 1.0 | 4.0 | 4.0 | 2.0 |
| �� | 8.0 | Vx | 4.0 | 4.0 | 2.0 |
��2����֪ij�����£�Ũ��c��S2O2-8������Ӧʱ��t�ı仯������ͼ�������������������䣬���ڴ������ͼ�У��ֱ����ͷ�Ӧ�¶Ⱥͼ������ʱc��S2O2-8����t�ı仯����ʾ��ͼ��������Ӧ�ı�ע����
��3����KI��Na2S2O3����۵Ļ����Һ�м���һ������K2S2O8��Һ������Һ�е�S2O32-��Na2S2O3�ľ�����Һ��ɫ������ɫ��Ϊ��ɫ��Ϊȷ���ܹ۲쵽��ɫ��S2O2-3��S2O2-8��ʼ�����ʵ���������Ĺ�ϵΪ��n��S2O2-3����n��S2O2-8����2��1��
��4����Ҳ����������������Դ--﮵��صIJ��ϣ�
�õ�ط�ӦΪ��2Li��s��+I2��s���T2LiI��s����H
��֪��
4Li��s��+O2��g���T2Li2O��s����H1
4LiI��s��+O2��g���T2I2 ��s��+2Li2O��s����H2
���ط�Ӧ�ġ�H=$\frac{��{H}_{1}-��{H}_{1}}{2}$����缫��Ϊ�õ�ص�������

 ����A��B��C��D��E���ֶ����ڷǽ���Ԫ�أ���ԭ��������������A��ԭ�Ӱ뾶��С��Ԫ�أ�BԪ�ص�һ�ֺ��ؿ����ڿ��Ŷϴ���DԪ�ص�s�����p����ϵĵ�������ȣ�C��E��ͬ����Ԫ�أ����ǵĺ������3��δ�ɶԵ��ӣ���ش��������⣺
����A��B��C��D��E���ֶ����ڷǽ���Ԫ�أ���ԭ��������������A��ԭ�Ӱ뾶��С��Ԫ�أ�BԪ�ص�һ�ֺ��ؿ����ڿ��Ŷϴ���DԪ�ص�s�����p����ϵĵ�������ȣ�C��E��ͬ����Ԫ�أ����ǵĺ������3��δ�ɶԵ��ӣ���ش��������⣺