��Ŀ����
����A��B��C��D��E���ֶ�����Ԫ�أ����ǵĺ˵�ɰ� C��A��D��E��˳������C��D���ֱܷ���A��ԭ�Ӹ�����1��1��2��1�γɻ����CB����EA2��Ӧ����C2A����̬����EB4��
��д������Ԫ������A B ,C ��D , E ��
�ƻ���E��ԭ�ӽṹ��ͼ ��д������ʽD2A2 , EB4 ��
�DZȽ�EA2��EB4���۵�ߵ� < ��
��д��D������CuSO4��Һ��Ӧ�����ӷ��� ��
�����������⣬�ƣ��� ��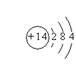 ��
��
�� SiO2 >SiF4
�� 2Na+2H2O+Cu2+=Cu(OH)2��+2Na++H2��
����:
���⿼������Ԫ�صĻ��ϼ���ԭ�������Ĺ�ϵ���Լ��й�Ԫ�����ڱ��и���ϵʽ�ľ���Ӧ�á�����Ĺؼ����ڷ���EB4��EԪ��ֻ���Ǣ�A��Ԫ��C��Si����B ��ԭ����������С����B������ΪHԪ�أ�E�ļ�̬ӦΪ+4��BӦΪ��A��Ԫ�أ���ֻ��ΪF�������ClԪ�أ���ԭ��������E����Eֻ��ΪSi����EB4ΪSiF4, ��CB�Ļ��������ʽ��֪CΪ+1�ۣ�����C2A��֪AΪ-2�ۣ�ֻ��ΪO������O��ԭ�Ӹ�����1��1��2��1�γɻ������Ԫ��ֻ����H��Na��



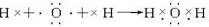



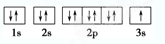
 NH4����OH�������ж�����ˮ���γɵĺ����ṹ��________��(����ͼ�е���ĸ)��
NH4����OH�������ж�����ˮ���γɵĺ����ṹ��________��(����ͼ�е���ĸ)�� 