��Ŀ����
6��������KSCN��SCN-�ǡ���±���ӡ�����Һ�������Եĺ���Fe3+����Һ�У���Һ��ɺ�ɫ�����ú�ɫ��Һ��Ϊ���ݣ���һ���м�������KMnO4��Һ����ɫ��ȥ��������һ����ͨ��SO2����ɫҲ��ȥ������˵������ȷ���ǣ�������| A�� | �ٺ�ɫ��ȥ��ԭ����KMnO4��SCN-������ʹFe��SCN��3��ʧ | |
| B�� | �ں�ɫ��ȥ��ԭ����SO2��Fe3+��ԭΪFe2+ | |
| C�� | �ں�ɫ��ȥ��ԭ����SO2��SCN-��ԭ | |
| D�� | SCN-���ʵ������¿�ʧȥ���ӱ�����������Ϊ��SCN��2 |
���� A�����ݸ�����ؾ���ǿ�����ԣ����Խ�SCN-������ԭ�����жϣ�
B�����ݶ���������л�ԭ�ԣ�Fe3+���������Խ��
C��SO2����Ԫ��Ϊ+4�ۣ�SCN-����̼Ԫ����+4�ۣ���Ԫ����-3�ۣ���Ԫ�صĻ��ϼ�Ϊ-2�ۣ�
D��������ؾ���ǿ�����ԣ����Խ���ԭ�Ե�����������
��� �⣺A����������������������ػᷢ����Ϸ�Ӧ��Fe3++3SCN-?Fe��SCN��3���Ժ�ɫ��������ؾ���ǿ�����ԣ����Խ�SCN-������ʹ[��ɫ��ʧ����A��ȷ��
B��Fe3+���������ԣ�����������л�ԭ�ԣ���Ӧ������������ӡ��������Ӻ������Ӣ��к�ɫ��ȥ����ӦΪ��2Fe3++SO2+2H2O=2Fe2++4H++SO42-����B��ȷ��
C��SO2����Ԫ��Ϊ+4�ۣ������ԭSCN-������������е���Ϊ+6�ۣ���SCN��������ͣ���SCN-��-1���Ѿ�Ϊ��ͼۣ����ٽ��ͣ�̼Ԫ����+4�ۣ���������+4�۵���Ԫ����-3�ۣ����ϼ۲����ٽ��ͣ���Ԫ�صĻ��ϼ�Ϊ-2�ۣ�Ҳ��������+4�۵������Զ���SCN������ԭ���Ż�������ԭ�ӣ�����������������C����
D��������ؾ���ǿ�����ԣ����Խ�SCN-����Ϊ��SCN��2���൱��±�ص���X2����D��ȷ��
��ѡC��
���� ������Ҫ���������������ӵļ��飬���ո�����ص��������ǽ����Ĺؼ�����Ŀ�ѶȲ���

 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�| A�� | HCl�ĵ���ʽH��Cl | B�� | HClO�ĽṹʽH-Cl-O | ||
| C�� | MgBr2���γɹ����õ���ʽ��ʾΪ�� | D�� | Cl-�Ľṹʾ��ͼ |
| ���Ӵ��� | A | B | C | D | E | F | G |
| ԭ�Ӻ��� | ���� | ���� | ˫�� | ��� | ���� | ��� | ��� |
| ����� | 0 | 1+ | 1- | 0 | 2+ | 1+ | 0 |
��1��A���ӵĽṹʾ��ͼ
 ��G�Ļ�ѧʽ��CH4��
��G�Ļ�ѧʽ��CH4����2���Ƚ�BC��EC2�ļ���ǿ��BC��EC2���������=����
��3��F��C��Ӧ����D�����ӷ���ʽNH4++OH-=NH3��H2O��
CH3CH2CH2CH3��g��+6.5O2��g��=4CO2��g��+5H2O��l����H=-2878kJ/mol
��CH3��2CHCH3��g��+6.5O2��g��=4CO2��g��+5H2O��l����H=-2869kJ/mol
����˵����ȷ���ǣ�������
| A�� | ��������ȶ��Դ����춡�� | |
| B�� | �춡������е�̼�����������Ķ� | |
| C�� | �����ʵ��������������������춡������ | |
| D�� | �춡��ת��Ϊ������Ĺ�����һ�����ȹ��� |
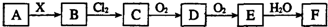
����˵���в���ȷ���ǣ�������
| A�� | ��X��ǿ��ʱ��A��B��C��D��E��F�о���ͬһ��Ԫ�أ�F������H2SO4 | |
| B�� | ��X��ǿ��ʱ��A��B��C��D��E��F�о���ͬһ��Ԫ�أ�F��HNO3 | |
| C�� | B��Cl2�ķ�Ӧ��������ԭ��Ӧ | |
| D�� | ��X��ǿ��ʱ��C�ڳ���������̬���� |
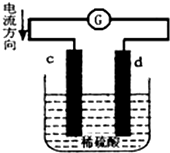 ��пƬ��ͭƬͬʱ����100mL ϡ�����У���ɵ�ԭ���װ����ͼ��ʾ��c��dΪ�����缫����ع�����������Һ������䣩�������й��жϲ���ȷ���ǣ�������
��пƬ��ͭƬͬʱ����100mL ϡ�����У���ɵ�ԭ���װ����ͼ��ʾ��c��dΪ�����缫����ع�����������Һ������䣩�������й��жϲ���ȷ���ǣ�������| A�� | ����·��ͨ��2 mol ����ʱ��d ����������22.4 L H2 | |
| B�� | ����·��ͨ��0.1 mol ����ʱ����Һ��c��Zn2+��=0.5 mol•L-1 | |
| C�� | cΪ����������������Ӧ | |
| D�� | ��ع�����ɺ���Һ��SO42-Ũ�Ȼ������� |

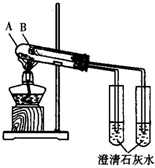
��1����μ���װ�õ������ԣ���C��������ĩ�˽���ˮ���У�����A��Բ����ƿ������ĩ�˳������ݣ�ֹͣ���Ⱥ�ĩ�˳���һ��ˮ��
��2��д������ˮ������Ӧ�Ļ�ѧ����ʽ3Fe+4H2O��g��$\frac{\underline{\;����\;}}{\;}$Fe3O4+4H2
��3����֤�����������Ԫ�صļ�̬
��ѡʵ���������Լ����ձ����Թܡ���������ҩ�ס��ιܡ��ƾ��ơ��ԹܼУ�1mol/L CuSO4��3mol/L H2SO4��3mol/L HNO3��30%H2O2��0.01mol/L KMnO4��20%KSCN������ˮ��
�ڴ���ϰ��±��ĸ�ʽд��ʵ�鲽�衢Ԥ����������ۣ�
| ʵ�鲽�� | Ԥ����������� | |
| ����1 | ȡ��Ӧ�����Ĺ���ag���Թ��У�����������1mol/L CuSO4��Һ�����������Һ���롢ϴ�Ӻ���������еμ�������3mol/LH2SO4�����ܽ⣬���˺���Һ���250mL��Һ�����ã� | - |
| ����2 | ȡ��������1����Һ���Թ��У��μ�1��2��20%KSCN | ��Һ���ɫ���������ﺬ+3���� |
| ����3 | ȡ��������1����Һ���Թ��У��μ�1��2��0.01mol/LKMnO4 | ��Һ��ɫ��ȥ���������ﺬ+2���� |
��4�������������Ԫ�ص����������IJⶨ�ɲ���ͼ2�����̣����в����������Һ�м���п������ɫ�պ���ʧ���������0.010mol/LKMnO4��Һ�ζ����յ㣬����KMnO4��Һƽ��Ϊv mL������֪5Fe2++MnO4-+8H+�T5Fe3++Mn2++4H2O�����м���п�۵�Ŀ���ǽ�Fe3+��ԭΪFe2+��ʵ����ag��Ʒ�й��������Ԫ�ص���������Ϊ$\frac{250��5��56g/mol��0.010mol/L��V��1{0}^{-3}L}{25a}$��100%_��ֻ�м������ʽ����
 �о�Cl2��SO2��CO2��NH3����������ʶ���������������Ҫ�����壮
�о�Cl2��SO2��CO2��NH3����������ʶ���������������Ҫ�����壮