��Ŀ����
3����1��ij�¶�ʱ����2L�����У�X��Y��Z�������ʵ����ʵ�����ʱ��仯����ͼ��ʾ����ͼ�����ݷ������ÿ��淴Ӧ�Ļ�ѧ����ʽΪ��3X+Y?2Z����Ӧ��ʼ��2min��Z��ƽ����Ӧ����Ϊ0.05mol/L•min��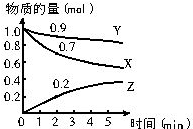
��2����ͬʱ�������·�Ӧ���ʣ�
��v��X��=0.075mol/��L•min������v��Y��=0.001mol/��L•s��
��v��Z��=0.06mol/��L•min�������ɴ�С��ϵ��ȷΪ���ڣ��ۣ��٣����ţ�
���� ��1������ͼ��XY���ʵ�����СΪ��Ӧ�Z���ʵ�������Ϊ�������Ӧ�����ʵ���֮�ȵ��ڻ�ѧ����ʽ������֮�ȵõ���ѧ����ʽ����Ӧ����V=$\frac{��c}{��t}$��
��2�����ݻ�ѧ��Ӧ����֮�ȵ��ڻ�ѧ����ʽ������֮�ȣ�����Ϊͬһ�����ʵ��������ȽϷ�Ӧ������
��� �⣺��1������ͼ��XY���ʵ�����СΪ��Ӧ�Z���ʵ�������Ϊ�����X�ı仯���ʵ���=1.0mol-0.7mol=0.3mol��Y�仯�����ʵ���=1.0mol-0.9mol=0.1mol��Z�������ʵ���Ϊ0.2mol����Ӧ�����ʵ���֮�ȵ��ڻ�ѧ����ʽ������֮��=0.3����01��0.2=3��1��2����Ӧ�Ļ�ѧ����ʽΪ��3X+Y?2Z����Ӧ��ʼ��2min��Z��ƽ����Ӧ����Ϊ=$\frac{\frac{0.2mol}{2L}}{2min}$=0.05mol/L•min��
�ʴ�Ϊ��3X+Y?2Z��0.05mol/L•min��
��2����ѧ����ʽΪ��3X+Y?2Z�����ݷ�Ӧ����֮�ȵ��ڻ�ѧ����ʽ������֮�ȣ�
��v��X��=0.075mol/��L•min����
��v��Y��=0.001mol/��L•s����v��X��=3v��Y��=3��0.075mol/��L•min��=0.225mol/L•s��
��v��Z��=0.06mol/��L•min����v��X��=$\frac{3}{2}$v��Z��=$\frac{3}{2}$��0.06mol/��L•min��=0.09mol/��L•min����
��Ӧ���ʴ�СΪ���ڣ��ۣ��٣�
�ʴ�Ϊ
���� ���⿼���˻�ѧƽ��Ľ�����ͼ��������㣬��ѧ����ʽ��д����Ӧ���ʵļ���Ӧ�úʹ�С�Ƚϣ����ո���ʵ���ǽ���ؼ�����Ŀ�ϼ�

| A�� | -483.6kJ•mol-1 | B�� | -241.8kJ•mol-1 | C�� | -120.6kJ•mol-1 | D�� | +241.8kJ•mol-1 |
 ����Fe2+��I-��Br-����Һ��ͨ��������������Һ�и������ӵ����ʵ����仯��ͼ��ʾ���й�˵������ȷ���ǣ�������
����Fe2+��I-��Br-����Һ��ͨ��������������Һ�и������ӵ����ʵ����仯��ͼ��ʾ���й�˵������ȷ���ǣ�������| A�� | �߶�BC����Fe3+���ʵ����ı仯��� | |
| B�� | ԭ�����Һ��n��FeBr2��=3mol | |
| C�� | ��ͨ��2molCl2ʱ����Һ���ѷ��������ӷ�ӦΪ��2Fe2++2I-+2Cl2=2Fe3++I2+4Cl- | |
| D�� | ԭ��Һ��n��Fe2+����n��I-����n��Br-��=2��1��3 |
| A�� | 12�� | B�� | 10�� | C�� | 8�� | D�� | 14�� |
 +3Fe+6HCl��
+3Fe+6HCl�� +3FeCl2+2H2O�����ﱽ����ԭ��ǿ���ױ����������ɼױ��ϳɶ���������IJ���������ǣ�������
+3FeCl2+2H2O�����ﱽ����ԭ��ǿ���ױ����������ɼױ��ϳɶ���������IJ���������ǣ�������| A�� | �ױ�$\stackrel{����}{��}$X$\stackrel{������}{��}$Y$\stackrel{��ԭ����}{��}$���������� | |
| B�� | �ױ�$\stackrel{������}{��}$X$\stackrel{����}{��}$Y$\stackrel{��ԭ����}{��}$���������� | |
| C�� | �ױ�$\stackrel{��ԭ}{��}$X$\stackrel{������}{��}$Y$\stackrel{����}{��}$���������� | |
| D�� | �ױ�$\stackrel{����}{��}$X$\stackrel{��ԭ����}{��}$Y$\stackrel{������}{��}$���������� |

| A�� | ����Na2CO3��Һ��CH3COOC2H5��ѡ�� | B�� | ��CCl4��ȡ��ˮ�еĵ⣬ѡ�� | ||
| C�� | ʵ����������ˮ����ȡ��ѡ�� | D�� | �����ᴿ��ѡ�ں͢� |
| A�� | �ⶨ��ҺpHʱ��pH��ֽ��������ˮ��ʪ | |
| B�� | ����ʵ���У�����ǰ������������ƿ�м������Ƭ | |
| C�� | ������ζ��ζ���ˮʵ���У��ü�����ָʾ���Լ�Сʵ����� | |
| D�� |  ����ͼ��ʾ�ķ����ų���ʽ�ζ��ܽ����е����� ����ͼ��ʾ�ķ����ų���ʽ�ζ��ܽ����е����� |
| A�� | Fe2O3��Fe3O4��FeO | B�� | FeO��Fe3O4 | C�� | Fe3O4��Fe2O3 | D�� | FeO��Fe2O3 |