��Ŀ����
ʵ������Ҫ0.1mol/LNaOH��Һ450mL ��0.5mol/L������Һ500mL.������������Һ����������ش��������⣺
(1)��ͼ��ʾ��������������Һ�϶�����Ҫ���� ______������ţ�������������Һ�����õ��IJ���������________�����������ƣ���
(2)���в����У�����ƿ�����߱��Ĺ�����_________������ţ���
A.����һ�����ȷŨ�ȵı���Һ
B��������Һ
C����������ƿ������µ����������Һ��
D��ȷϡ��ijһŨ�ȵ���Һ
E�����������ܽ��������
(3)���ݼ�����������ƽ��ȡNaOH������Ϊ__________g������ʱ������ȷ�IJ���˳������ĸ��ʾ��
ÿ����ĸֻ����һ�Σ�____________��
A����30mLˮϴ���ձ�2-3�Σ�ϴ��Һ��ע������ƿ
B��ȷ��ȡ���������������ƹ������ձ��У��ټ�������ˮ(Լ30mL)���ò���������������ʹ�����ܽ�
C�����ܽ������������Һ�ز�����ע������ƿ��
D.������ƿ�ǽ�����ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�����
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�2��3cm��
(4)���ݼ����֪��������������Ϊ98%���ܶ�Ϊ1.84g/ ��Ũ��������Ϊ��__________mL(����
��Ũ��������Ϊ��__________mL(����
�������һλС��)�����ʵ������15mL��20mL��50mL��Ͳ��Ӧѡ��____________mL����Ͳ��á�
(1)A��C��1�֣���ȫ�ԲŸ�1�֣� �ձ�����������2�֣� (2)B��C��E��1�֣�
��ȫ�ԲŸ�1�֣� (3)2.0��2�֣� BCAFED��2�֣� (4)13.6��2�֣� 15 ��1�֣�
���������������1������һ�����ʵ���Ũ��ʱ���������У�������ƽ����Ͳ��500mL����ƿ���ձ�������������ͷ�ιܡ�ҩ�ȣ���ƿ�ͷ�Һ©������Ҫ�����Դ�ѡA��C������û��500ml����ƿ����������450mL��Һ����Ҫ500mL����ƿ�������Ҫ�ܽ��ϡ��ʱ�õ����ձ��Ͳ�������
��2������ƿֻ����������һ�����ȷŨ�ȵ���Һ���������ƻ��������ƿ������µ����������Һ�壬����ϡ�ͻ��ܽ�ҩƷ���������������ܽ�������ʣ����Դ�ѡB��C��E��
��3����Ҫ�������Ƶ�������0.5L��0.1mol/L��40g/mol��2.0g�����Ʋ�����Ҫ�м��㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�������ȷ��ȡ��������Na2CO3�������ձ��У��ټ�������ˮ���ò���������������ʹ�����ܽ⣬����ȴ�����£����ܽ��Na2CO3��Һ�ز�����ע��1000mL������ƿ�У�������ˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1��2cm�������ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶����У�������ƿ�ǽ�����ҡ�ȼ��ɣ�ʵ����ȷ�IJ���˳����BCAFED��
��4��Ũ�����Ũ��c��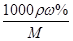 ��
��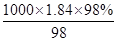 mol/L��18.4mol/L��Ũ����ϡ��ǰ�����ʵ����ʵ������䣬��Ũ��������ΪV������18.4mol/L��V��0.5/L��0.5L�����V��0.0136L=13.6mL��ѡȡ����Ͳ���Ӧ�õ��ڻ������ȡ��Һ�������ѡ15mL��Ͳ��
mol/L��18.4mol/L��Ũ����ϡ��ǰ�����ʵ����ʵ������䣬��Ũ��������ΪV������18.4mol/L��V��0.5/L��0.5L�����V��0.0136L=13.6mL��ѡȡ����Ͳ���Ӧ�õ��ڻ������ȡ��Һ�������ѡ15mL��Ͳ��
���㣺����һ�����ʵ���Ũ�ȵ�����

 53���ò�ϵ�д�
53���ò�ϵ�д���11�֣��ס�����λͬѧ�ֱ��ò�ͬ�ķ�������100mL 3.6mol/L��ϡ���ᡣ
��1��������18mol/L��Ũ����������Һ����Ҫ�õ�Ũ��������Ϊ ��
��2����ѧ������ȡŨ���ᣬС�ĵص���ʢ������ˮ���ձ��У�������ȣ�����ȴ�����º�ת�Ƶ�100 mL ����ƿ�У���������ˮ���ձ�������ϴ��2��3�Σ�ÿ��ϴ��ҺҲת�Ƶ�����ƿ�У�Ȼ��С�ĵ�������ƿ����ˮ���̶��߶��ݣ�����ƿ�����������µߵ�ҡ�ȡ�
�ٽ���Һת�Ƶ�����ƿ�е���ȷ������ ��
��ϴ�Ӳ����У���ϴ���ձ����ϴҺҲע������ƿ����Ŀ����__ _______��
���ý�ͷ�ι�������ƿ�м�ˮʱ����С��Һ�泬���˿̶ȣ�
�����ķ�����_ ___������ţ���
| A����������Һ�壬ʹ��Һ����̶������� |
| B��С�ļ�������ƿ����������ʹ��Һ����̶������� |
| C�����������һ������Ũ���� |
| D���������� |
��4�������ƹ�������������в�����������Һ��Ŀ����Һ��ȣ�Ũ�Ƚ���α仯�����á�ƫ����ƫС������Ӱ�족��գ�
������Ͳ��ȡŨ����ʱ���Ӷ�������������Һ�����ʵ���Ũ��______;
�ڶ���ʱ����������ƿ�̶��ߣ���������Һ�����ʵ���Ũ��______;
ijһ��θҩ�е������Ϊ̼���,��������������������IJⶨ����:
��������0.1 mol��L-1��HCl��Һ��0.1 mol��L-1��NaOH��Һ
��ȡһ��(ҩƬ������ͬ) 0.2 g�Ĵ�θҩƬ,ĥ������20.0 mL����ˮ
���Է�̪Ϊָʾ��,��0.1 mol��L-1��NaOH��Һ�ζ�,��ȥV mL��ζ��յ�
�ܼ���25 mL 0.1 mol��L-1��HCl��Һ
(1)д��ʵ����̵IJ���(д���˳��)������������������������
(2)��ͼ��ʾ����������0.1 mol��L-1��HCl��Һ��0.1 mol��L-1��NaOH��Һ�϶�����Ҫ��������(�����)������,����������Һ����Ҫ�IJ���������������(����������)�� 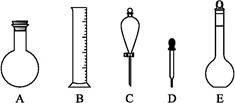
(3)����������ҺӦѡ�õ�����ƿ�����(����ĸ)������������
| A��50 mL��50 mL |
| B��100 mL��100 mL |
| C��100 mL��150 mL |
| D��250 mL��250 mL |
(5)ÿ��θҩ�к�̼��Ƶ�����Ϊ������ g��
ij��ʵ������0.4 mol/L NaOH��Һ480 mL�����Ʒ������£�
��1�����Ƹ���ҺӦѡ��_______________mL����ƿ��
��2����������ƽȷ����__________g ����NaOH��
��3���������õ�NaOH�������500 mL���ձ��У�����Լ300 mL����ˮ���ò��������裬ʹ����ȫ���ܽ⣬��__________________���ձ��е���Һע������ƿ�У�����������ˮϴ���ձ� �Σ�ϴ�Ӻ����Һһ��ת������ƿ������ζ�����ƿ��ʹ��Һ��Ͼ��ȣ�������ƿ�м�������ˮ����Һ��_____________________ʱ������__________������ˮ��Һ����͵���̶������С��Ǻ�ƿ�������µߵ���Ͼ��ȣ��������ƹ����г��������������ʹ�����Ƶ�NaOH��Һ��Ũ��ƫ�ߵ���___________��ƫ�͵���___________����ʵ����û��Ӱ�����___________�����ѡ�����ţ���
| A�����õ�NaOH�л�������Na2O |
| B����������ƽ����һ����������NaOHʱ�����õ�С�ձ��ڱڲ�̫���� |
| C��������Һ���õ�����ƿϴ����û�к�� |
| D������NaOH���ձ����ܽ����������Һת�Ƶ�����ƿ�ڲ����Ž��к������� |
F�����ȷ��NaOH��Һ��������ݣ�ʱ�����ӹ۲�Һ��������ƿ�̶���
G������ҡ�Ⱥ�ֹ������Һ����ڿ̶��ߣ��ټ�����ˮ���̶��ߡ�
�����йظ���˵����ȷ����
| A��D2��T2��H2��Ϊͬ�������� |
| B��������춡�黥Ϊͬϵ�� |
| C��ʯī�����ʯ��Ϊͬλ�� |
| D���������ϩ��Ϊͬ���칹�� |
��ѧ���ѧ�����������ͻ���������ء������й�˵���д������
| A��̫���ܵ�ذ��еĹ裬��Ԫ�����ڱ��д��ڽ�����ǽ����Ľ���λ�� |
| B��������ɱ��H7N9�������в���������Ϊ�����ĵ��������ȱ��� |
| C��ú��������Һ�����������Խ�����Ⱦ |
| D��PM2.5��ָ������ֱ��С�ڻ����2.5�Ŀ����PM2.5�Ƚ�������С������ȱ�����ʿ������ؽ������ӣ���κ����������ܴ��Σ�� |