��Ŀ����
����Ŀ���ش����и��⣺
��1��CH![]() ����CH3��CH
����CH3��CH![]() ������Ҫ���л���Ӧ�м��壬���ǵĵ���ʽ�ֱ���________��________��________������CH
������Ҫ���л���Ӧ�м��壬���ǵĵ���ʽ�ֱ���________��________��________������CH![]() �ļ���Ӧ��________��
�ļ���Ӧ��________��
��2�������������ڻ�ѧ��ҵ������ҪӦ�á�N![]() �����������ӣ���д��������ԭ�ӹ��ɵĺ�����N
�����������ӣ���д��������ԭ�ӹ��ɵĺ�����N![]() �ĵ�������ͬ�ķ��ӵĽṹʽ_______________��______________��
�ĵ�������ͬ�ķ��ӵĽṹʽ_______________��______________��
��3��SiO![]() ��SO3��NO
��SO3��NO![]() ���������ǵȵ����壬���ǵ����幹��Ϊ____________������SiO44-��PO43-��SO42-�����幹��Ϊ_____________��
���������ǵȵ����壬���ǵ����幹��Ϊ____________������SiO44-��PO43-��SO42-�����幹��Ϊ_____________��
��4��C2O42-��________�ǵȵ����壬C2O42-���н�ǿ�Ļ�ԭ�ԣ���ʹ����KMnO4��Һ��ɫ��д����Ӧ�����ӷ���ʽ__________________________________________��
��5��2ԭ��14���ӵĵȵ�����Ĺ�ͬ�ص��������ж����й�����������ٳ���Ӧ��3������______________��______________��______________(���ӻ�����)��ÿ�����ӻ����Ӿ�����_________��������________��������
���𰸡� 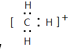
![]()
![]() 120�� O=C=O N=N=O ƽ�������� ���������� N2O4 5C2O42-+2MnO4-+16H+=10CO2��+2Mn2++8H2O N2 CO CN-��C22- 1 2
120�� O=C=O N=N=O ƽ�������� ���������� N2O4 5C2O42-+2MnO4-+16H+=10CO2��+2Mn2++8H2O N2 CO CN-��C22- 1 2
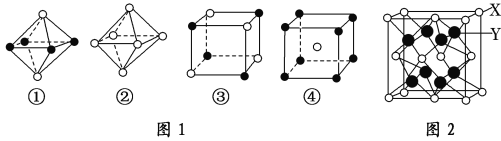
����������1������������������Լ����еĻ�ѧ����𣬸��ݿռ乹���жϼ��ǣ�
��2���������ӵĵ�������22��
��3����Ϊ�ȵ���������ṹ���ƣ�
��4��ԭ�����ͼ۵������ֱ���ȵĻ�Ϊ�ȵ����壻���ݵ��ӵ�ʧ�غ���ƽ����д����ʽ��
��5�����ݵȵ�����ĺ����𣻸�����������һ�����������������жϡ�
��1��CH3����1������ɣ����й��ۼ�������ʽΪ ����CH3������ɣ����й��ۼ�������ʽΪ
����CH3������ɣ����й��ۼ�������ʽΪ![]() ��CH3����1������ɣ����й��ۼ�������ʽΪ
��CH3����1������ɣ����й��ۼ�������ʽΪ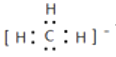 ��CH3��������ԭ�ӵļ۲���Ӷ�����3+(4-1��3��1)/2=3������ƽ�������νṹ�����Լ���Ӧ��120�㡣
��CH3��������ԭ�ӵļ۲���Ӷ�����3+(4-1��3��1)/2=3������ƽ�������νṹ�����Լ���Ӧ��120�㡣
��2���������ӵĵ�������22����������ԭ�ӹ��ɵĺ�����N3���ĵ�������ͬ�ķ��ӵĽṹʽΪO=C=O��N=N=O��
��3������������ƽ�������νṹ��SiO32����SO3��NO3�����������ǵȵ����壬���ǵĽṹ���ƣ������ǵ����幹��Ϊƽ�������Σ�SiO44-��PO43-��SO42-��Ϊ�ȵ����壬�����������ԭ�ӵļ۲���Ӷ�����4+(6+2-2��4)/2=4�����幹��Ϊ���������Ρ�
��4��C2O42-����6��ԭ�Ӻ�34���۵��ӣ����N2O4�ǵȵ����壻C2O42-���н�ǿ�Ļ�ԭ�ԣ���ʹ����KMnO4��Һ��ɫ�����������Ƕ�����̼����ԭ�����������ӣ����ݵ��ӵ�ʧ�غ㡢ԭ���غ�͵���غ��֪��Ӧ�����ӷ���ʽΪ5C2O42-+2MnO4-+16H+��10CO2��+2Mn2++8H2O��
��5��2ԭ��14���ӵĵȵ�����Ĺ�ͬ�ص��������ж����й�������������N2��CO��CN-��C22-�ȡ�������������һ���������������������ÿ�����ӻ����Ӿ�����1��������2��������