��Ŀ����
����Ŀ���ش����и��⣺
��1����֪FeCl3�ķе㣺319�棬�۵㣺306�棬��FeCl3�ľ�������Ϊ___________��P�γɵ��������������ǿ������˳��Ϊ��HPO3>H3PO4>H3PO3��ԭ����___________________��
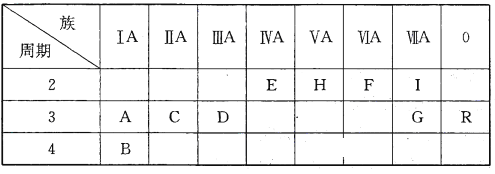
��2��ԭ������С��36��Ԫ��Q��T�������ڱ��мȴ���ͬһ������λ��ͬһ�壬��ԭ������T��Q��2��T�Ļ�̬ԭ�Ӽ۵����Ų�ʽΪ___________________��Q2����δ�ɶԵ�������______________��
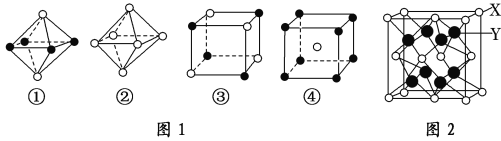
��3����ͼ1�Ǵ�NaCl��CsCl����ṹͼ�зָ�����IJ��ֽṹͼ���ж�NaCl����ṹ��ͼ��ͼ1�е�______________��
��4��[Cu(NH3)4]2���������д��ڵĻ�ѧ��������_____________(�����)��
����λ�� �ڽ����� �ۼ��Թ��ۼ� �ܷǼ��Թ��ۼ� �����Ӽ� �����
��5����[Cu(NH3)4]2�����жԳƵĿռ乹�ͣ��ҵ�[Cu(NH3)4]2���е�����NH3������Cl��ȡ��ʱ���ܵõ����ֲ�ͬ�ṹ�IJ����[Cu(NH3)4]2���Ŀռ乹��Ϊ______________(�����)
��ƽ�������� ���������� �������� ��V��
��6��X��Y���γ����ӻ�����侧���ṹ��ͼ2��ʾ������X��Y�����ԭ�������ֱ�Ϊa��b��NA��ʾ�����ӵ������������ܶ�Ϊ��g/cm3�����о��������X��Y֮��ĺ˼������_____________pm���ú��ѡ�a��b��NA�Ĵ���ʽ�����
���𰸡� ���Ӿ��� ������������У����ǻ�����ĿԽ�࣬����Խǿ 3d84s2 4 �� �٢� �� ![]()
����������1���Ȼ����۷е�ϵͣ�ӦΪ���Ӿ��壻ͬ��Ԫ�غ����ᣬ��Ԫ�ػ��ϼ�Խ�ߣ�������Խǿ�����ǻ���Խ�࣬����Խǿ��
��2��ԭ������С��36��Ԫ��Q��T�������ڱ��мȴ���ͬһ������λ��ͬһ�壬��ԭ������T��Q��2����QΪFe��TΪNi��
��3��NaCl�����������壬ÿ����������Χ��6�������ӡ�ÿ����������Χ��6�������ӣ�
��4��[Cu(NH3)4]2����ͭ�����뵪ԭ��֮���γ���λ��������������Nԭ����Hԭ��֮���γɼ��Լ���
��5��[Cu(NH3)4]2���е�����NH3������Cl��ȡ���ܵõ����ֲ�ͬ�ṹ�IJ��[Cu(NH3)4]2���Ŀռ乹��Ϊƽ�������Σ�
��6�����ݾ�̯�����㾧����X��Yԭ����Ŀ���������㾧��������������m=��V���㾧��������������㾧���ⳤx��Yԭ������Χ4��Xԭ���γ���������ṹ���ݴ˽��
��1��FeCl3�ķе㣺319�棬�۵㣺306�棬�۷е�ϵͣ�Ӧ���ڷ��Ӿ��壻H3PO4��HPO3��H3PO3��PԪ�ػ��ϼ�����Ϊ+5��+5��+3��H3PO4��HPO3�з��ǻ�������Ϊ1��2��Ԫ�ػ��ϼ�Խ�ߣ�������Խǿ�����ǻ���Խ�࣬����Խǿ��������HPO3��H3PO4��H3PO3��
��2��ԭ������С��36��Ԫ��Q��T�������ڱ��мȴ���ͬһ������λ��ͬһ�壬��ԭ������T��Q��2����QΪFe��TΪNi��Ni�Ļ�̬ԭ����Χ���ӣ��۵��ӣ��Ų�ʽΪ3d84s2��Fe2+�ĺ�������Ų�Ϊ1s22s22p63s23p63d6��3d�ܼ���4��δ�ɶԵ��ӣ�
��3��������NaCl�����У�ÿ��Na+��Χͬʱ����������ĵȾ����6��Cl-��ÿ��Cl-��Χͬʱ����������ĵȾ����6��Na+��ͼ����ѡȡ����һ�����ӣ�Ȼ����X��Y��Z�����и�õ�6���Ⱦ����������Ĵ��෴��ɵ����ӣ���������λ����6���۷���������
��4��[Cu(NH3)4]2����ͭ�����뵪ԭ��֮���γ���λ��������������Nԭ����Hԭ��֮���γɼ��Լ�����ѡ�٢ۣ�
��5��[Cu(NH3)4]2��������NH3������Cl-ȡ���ܵõ����ֲ�ͬ�ṹ�IJ�����[Cu(NH3)4]2���Ŀռ乹��Ϊƽ�������Σ���ѡ�٣�
��6��������Xԭ����ĿΪ8��1/8+6��1/2=4��Yԭ����ĿΪ8������������Ϊ![]() �������ܶ�Ϊ��g/cm3�������Ϊ
�������ܶ�Ϊ��g/cm3�������Ϊ![]() g����g/cm3���ʾ����ⳤx=
g����g/cm3���ʾ����ⳤx=![]() ��Yԭ������Χ4��Xԭ���γ���������ṹ����Y��X֮��ľ���Ϊy���������������ĵ��������ĵľ���Ϊy/3����������ĸ�Ϊ4y/3�����������ⳤ=
��Yԭ������Χ4��Xԭ���γ���������ṹ����Y��X֮��ľ���Ϊy���������������ĵ��������ĵľ���Ϊy/3����������ĸ�Ϊ4y/3�����������ⳤ=![]() ���������������ĸ�Ϊ
���������������ĸ�Ϊ![]() ���������ĵ��ߵľ���Ϊ
���������ĵ��ߵľ���Ϊ![]() ����
����![]() ��������y=
��������y=![]() ����Y��X�ľ���Ϊ
����Y��X�ľ���Ϊ![]() ��
��

 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�