��Ŀ����
һ����ѧ��Ӧһ�㶼�оɻ�ѧ�����ƻ����»�ѧ�����γɡ���һ�������£������������ϳɰ����������仯��������ͼ(a��û��ʹ�ô����ķ�Ӧ���̣�b��ʹ���˴����ķ�Ӧ����)������������ȷ����
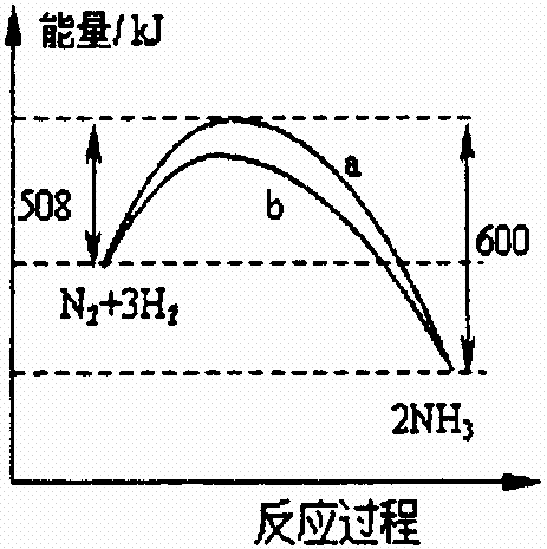 ��ʹ�ô����ɽ��������ܺ�
��ʹ�ô����ɽ��������ܺ�
�ڸ÷�Ӧ���Ȼ�ѧ����ʽΪN2(g)+3H2(g) ![]() 2NH3(g)+92kJ
2NH3(g)+92kJ
����ͬ״���£�lmol������3mol��������������2mol������������
����ͬ״���£�lmol������3mol�����ܼ��ܵ���2mol�������ܼ���
A���٢ڢۢ� B��ֻ�Тڢۢ� C��ֻ�Т٢ڢ� D��ֻ�Тڢ�

��10�֣���ѧ���ļ�����ָ��̬ԭ�Ӽ��γ�1mol��ѧ��ʱ�ͷŵ���������H(g)��I(g)��H��I(g) ��H ==��297KJ��H��I���ļ���Ϊ297KJ?mol��1��Ҳ��������Ϊ�ƻ�1mol H��I����Ҫ����297KJ��������һ����ѧ��Ӧһ�㶼�оɻ�ѧ�����ƻ����»�ѧ�����γɡ�
�±���һЩ�������ݣ�KJ?mol��1��:
| ���� |
| ���� |
| ���� |
| ���� |
H��H | 436 | H��F | 565 | C��F | 427 | C��O | 347 |
H��O | 464 | H��Cl | 432 | C��Cl | 330 | Cl��Cl | 243 |
H��S | 339 |
|
| C��I | 218 | S��S | 255 |
�ش��������⣺
(1)�ɱ��������ܷ�ó������Ľ��ۣ�
�ٰ뾶ԽС��ԭ���γɵĹ��ۼ�Խ�ι̣�������Խ��_______����ܡ����ܡ���
�ڷǽ�����Խǿ��ԭ���γɵĹ��ۼ�Խ�ι�________����ܡ����ܡ�����
�ܷ�������ҳ�һЩ���ɣ���д��һ����_________________ _______________����Ԥ��C��Br���ļ��ܷ�Χ_________<C��Br����<__________��
(2)���Ȼ�ѧ����ʽH2(g)��Cl2(g)=2HCl(g)����H= �D185KJ?mol��1����ϱ������ݿ���֪һ����ѧ��Ӧ�ķ�Ӧ�ȣ��跴Ӧ���������Ϊ��̬���뷴Ӧ���������ļ���֮��Ĺ�ϵ��___________________________�����Ȼ�ѧ����ʽ2H2(g)��S2(s)=2H2S(g)����H=-224.5KJ?mol��1�ͱ�����ֵ�ɼ����1mol S2(s)����ʱ��________������ա��ų�����_______KJ��������
�±���һЩ��������(kJ��mol-1)
��ѧ�� | ���� | ��ѧ�� | ���� | ��ѧ�� | ���� | ��ѧ�� | ���� |
H��H | 436 | Cl��Cl | 243 | H��Cl | 432 | H��O | 464 |
S=S | 255 | H��S | 339 | C��F | 427 | C��O | 347 |
C��Cl | 330 | C��I | 218 | H��F | 565 |
|
|
�ش��������⣺
(1)�ɱ��������ܷ�ó������Ľ��ۣ��ٰ뾶ԽС��ԭ���γɵĹ��ۼ�Խ�ι�(������Խ��)____________(��ܡ����ܡ�)���ڷǽ�����Խǿ��ԭ���γɵĹ��ۼ�Խ�ι�____________(��ܡ����ܡ�)���ܷ�������ҳ�һЩ���ɣ���д��һ����____________����Ԥ��C��Br���ļ��ܷ�Χ��____________��C��Br���ܣ�____________��
(2)���Ȼ�ѧ����ʽH2(g)+Cl2(g)====2HCI(g)����H=-185kJ��mol-1������ϱ������ݿ���֪һ����ѧ��Ӧ�ķ�Ӧ��(�跴Ӧ���������Ϊ��̬)�뷴Ӧ���������ļ���֮��Ĺ�ϵ��__________________________________________�����Ȼ�ѧ����ʽ2H2(g)+S2(s)=2H2S(g)����H=-224.5kJ��mol-1�ͱ�����ֵ�ɼ����lmolS2(s)����ʱ��____________(����ա��ų���)____________kJ��������
(3)±����RX��ͬ�������·�������ˮ�ⷴӦʱ��RF��RCl��RBr��RI(R��ͬ)�ķ�Ӧ�����ɴ�С��˳����________________��
 ��2009?������һģ��һ����ѧ��Ӧһ�㶼�оɻ�ѧ�����ƻ����»�ѧ�����γɣ���һ�������£������������ϳɰ����������仯��������ͼ��a��û��ʹ�ô����ķ�Ӧ���̣�b��ʹ���˴�
��2009?������һģ��һ����ѧ��Ӧһ�㶼�оɻ�ѧ�����ƻ����»�ѧ�����γɣ���һ�������£������������ϳɰ����������仯��������ͼ��a��û��ʹ�ô����ķ�Ӧ���̣�b��ʹ���˴� 2NH3��g��+92kJ
2NH3��g��+92kJ һ����ѧ��Ӧһ�㶼�оɻ�ѧ�����ƻ����»�ѧ�����γɣ���һ�������£������������ϳɰ����������仯��������ͼ��a��û��ʹ�ô����ķ�Ӧ���̣�b��ʹ���˴����ķ�Ӧ���̣�������������ȷ���ǣ�������
һ����ѧ��Ӧһ�㶼�оɻ�ѧ�����ƻ����»�ѧ�����γɣ���һ�������£������������ϳɰ����������仯��������ͼ��a��û��ʹ�ô����ķ�Ӧ���̣�b��ʹ���˴����ķ�Ӧ���̣�������������ȷ���ǣ�������