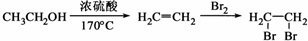ЬтФПФкШн
ЛЏКЯЮяGПЩвдЭЈЙ§BКЭFЗЂЩњЫѕОлЗДгІЖјжЦЕУЃЎЯжЭЈЙ§ШчЯТзЊЛЏЙиЯЕКЯГЩGЃК
ЦфжаЃК
ЂйAЪЧЗжзгЪНЮЊC8H10ЕФЗМЯуЬўЃЌжЛФмЩњГЩСНжжвЛфхДњЮяЃЌBгаЫсадЃЎ
Ђк0.01molЕФCеєЦјЃЈжЪСПЮЊ0.46gЃЉдкзуСПЕФбѕЦјжаЭъШЋШМЩеЃЌжЛЩњГЩ0.88g CO2КЭ0.54g H2OЃЎ
ЧыЭъГЩЯТСаЮЪЬтЃК
ЃЈ1ЃЉAЕФЪєгкЗМЯуЬўЭЌЗжвьЙЙЬхЕФНсЙЙМђЪНЮЊ
 ЃЌCЕФЗжзгЪНЮЊ
ЃЌCЕФЗжзгЪНЮЊ
ЃЈ2ЃЉаДГіЯТСаЗДгІЕФЛЏбЇЗНГЬЪНЃЈгаЛњЮягУНсЙЙМђЪНБэЪОЃЉЃКCЁњDЃК
ЃЈ3ЃЉжИГіЯТСаЗДгІЕФЗДгІРраЭЃКDЁњEЃК
ЃЈ4ЃЉCКЭFЕФЙиЯЕЪЧ
ЂйЭЌЯЕЮя ЂкЭЌЗжвьЙЙЬх ЂлЭЌРрЮяжЪ ЂмЭЌжжЮяжЪЃЎ

ЦфжаЃК
ЂйAЪЧЗжзгЪНЮЊC8H10ЕФЗМЯуЬўЃЌжЛФмЩњГЩСНжжвЛфхДњЮяЃЌBгаЫсадЃЎ
Ђк0.01molЕФCеєЦјЃЈжЪСПЮЊ0.46gЃЉдкзуСПЕФбѕЦјжаЭъШЋШМЩеЃЌжЛЩњГЩ0.88g CO2КЭ0.54g H2OЃЎ
ЧыЭъГЩЯТСаЮЪЬтЃК
ЃЈ1ЃЉAЕФЪєгкЗМЯуЬўЭЌЗжвьЙЙЬхЕФНсЙЙМђЪНЮЊ


C2H6O
C2H6O
ЃЎЃЈ2ЃЉаДГіЯТСаЗДгІЕФЛЏбЇЗНГЬЪНЃЈгаЛњЮягУНсЙЙМђЪНБэЪОЃЉЃКCЁњDЃК
CH3CH2OH
CH2=CH2+H2O
| ХЈСђЫс |
| 170Ёц |
CH3CH2OH
CH2=CH2+H2O
ЃЛB+FЁњGЃК| ХЈСђЫс |
| 170Ёц |
n +nHOCH2CH2OH
+nHOCH2CH2OH
 +ЃЈ2n-1ЃЉH2O
+ЃЈ2n-1ЃЉH2O
 +nHOCH2CH2OH
+nHOCH2CH2OH| ХЈСђЫс |
| Ёї |
 +ЃЈ2n-1ЃЉH2O
+ЃЈ2n-1ЃЉH2On +nHOCH2CH2OH
+nHOCH2CH2OH
 +ЃЈ2n-1ЃЉH2O
+ЃЈ2n-1ЃЉH2O
ЃЎ +nHOCH2CH2OH
+nHOCH2CH2OH| ХЈСђЫс |
| Ёї |
 +ЃЈ2n-1ЃЉH2O
+ЃЈ2n-1ЃЉH2OЃЈ3ЃЉжИГіЯТСаЗДгІЕФЗДгІРраЭЃКDЁњEЃК
МгГЩЗДгІ
МгГЩЗДгІ
ЃЛEЁњFЃКШЁДњЗДгІ
ШЁДњЗДгІ
ЃЎЃЈ4ЃЉCКЭFЕФЙиЯЕЪЧ
Ђл
Ђл
ЂйЭЌЯЕЮя ЂкЭЌЗжвьЙЙЬх ЂлЭЌРрЮяжЪ ЂмЭЌжжЮяжЪЃЎ
ЗжЮіЃКAЪЧЗжзгЪНЮЊC8H10ЕФЗМЯуЬўЃЌВЛБЅКЭЖШ=
=4ЃЌЙЪВрСДЮЊЭщЛљЃЌжЛФмЩњГЩСНжжвЛфхДњЮяЃЌдђAЮЊ ЃЌAБЛЫсадИпУЬЫсМибѕЛЏЩњГЩBЃЌBгаЫсадЃЌдђBЮЊ
ЃЌAБЛЫсадИпУЬЫсМибѕЛЏЩњГЩBЃЌBгаЫсадЃЌдђBЮЊ ЃЎ
ЃЎ
0.01molЕФCеєЦјЃЌжЪСПЮЊ0.46gЃЌдђЯрЖдЗжзгжЪСП=
=46ЃЌдкзуСПЕФбѕЦјжаЭъШЋШМЩеЃЌжЛЩњГЩ0.88g CO2КЭ0.54g H2OЃЌдђCЗжзгжаNЃЈCЃЉ=
=2ЁЂNЃЈHЃЉ=
=6ЃЌдђNЃЈOЃЉ=
=1ЃЌЙЪCЕФЗжзгЪНЮЊC2H6OЃЌгЩзЊЛЏЙиЯЕПЩжЊCЮЊCH3CH2OHЃЌЗЂЩњЯћШЅЗДгІЩњГЩDЃЌDЮЊCH2=CH2ЃЌввЯЉгыфхЗЂЩњМгГЩЗДгІЩњГЩEЃЌEЮЊBrCH2CH2BrЃЌ1ЃЌ2-ЖўфхввЭщЗЂЩњЫЎНтЗДгІЩњГЩFЃЌFЮЊHOCH2CH2OHЃЌгы ЗЂЩњЫѕОлЗДгІЩњГЩGЃЌGЮЊ
ЗЂЩњЫѕОлЗДгІЩњГЩGЃЌGЮЊ ЃЌОнДЫНтД№ЃЎ
ЃЌОнДЫНтД№ЃЎ
| 2ЁС8+2-10 |
| 2 |
 ЃЌAБЛЫсадИпУЬЫсМибѕЛЏЩњГЩBЃЌBгаЫсадЃЌдђBЮЊ
ЃЌAБЛЫсадИпУЬЫсМибѕЛЏЩњГЩBЃЌBгаЫсадЃЌдђBЮЊ ЃЎ
ЃЎ0.01molЕФCеєЦјЃЌжЪСПЮЊ0.46gЃЌдђЯрЖдЗжзгжЪСП=
| 0.46 |
| 0.01 |
| ||
| 0.01mol |
| ||
| 0.01mol |
| 46-12ЁС2-6 |
| 16 |
 ЗЂЩњЫѕОлЗДгІЩњГЩGЃЌGЮЊ
ЗЂЩњЫѕОлЗДгІЩњГЩGЃЌGЮЊ ЃЌОнДЫНтД№ЃЎ
ЃЌОнДЫНтД№ЃЎНтД№ЃКНтЃКAЪЧЗжзгЪНЮЊC8H10ЕФЗМЯуЬўЃЌВЛБЅКЭЖШ=
=4ЃЌЙЪВрСДЮЊЭщЛљЃЌжЛФмЩњГЩСНжжвЛфхДњЮяЃЌдђAЮЊ ЃЌAБЛЫсадИпУЬЫсМибѕЛЏЩњГЩBЃЌBгаЫсадЃЌдђBЮЊ
ЃЌAБЛЫсадИпУЬЫсМибѕЛЏЩњГЩBЃЌBгаЫсадЃЌдђBЮЊ ЃЎ
ЃЎ
0.01molЕФCеєЦјЃЌжЪСПЮЊ0.46gЃЌдђЯрЖдЗжзгжЪСП=
=46ЃЌдкзуСПЕФбѕЦјжаЭъШЋШМЩеЃЌжЛЩњГЩ0.88g CO2КЭ0.54g H2OЃЌдђCЗжзгжаNЃЈCЃЉ=
=2ЁЂNЃЈHЃЉ=
=6ЃЌдђNЃЈOЃЉ=
=1ЃЌЙЪCЕФЗжзгЪНЮЊC2H6OЃЌгЩзЊЛЏЙиЯЕПЩжЊCЮЊCH3CH2OHЃЌЗЂЩњЯћШЅЗДгІЩњГЩDЃЌDЮЊCH2=CH2ЃЌввЯЉгыфхЗЂЩњМгГЩЗДгІЩњГЩEЃЌEЮЊBrCH2CH2BrЃЌ1ЃЌ2-ЖўфхввЭщЗЂЩњЫЎНтЗДгІЩњГЩFЃЌFЮЊHOCH2CH2OHЃЌгы ЗЂЩњЫѕОлЗДгІЩњГЩGЃЌGЮЊ
ЗЂЩњЫѕОлЗДгІЩњГЩGЃЌGЮЊ ЃЌ
ЃЌ
ЃЈ1ЃЉAЮЊ ЃЌЪєгкЗМЯуЬўЭЌЗжвьЙЙЬхЕФНсЙЙМђЪНЮЊ
ЃЌЪєгкЗМЯуЬўЭЌЗжвьЙЙЬхЕФНсЙЙМђЪНЮЊ ЃЌгЩЩЯЪіЗжЮіПЩжЊCЕФЗжзгЪНЮЊC2H6OЃЌ
ЃЌгЩЩЯЪіЗжЮіПЩжЊCЕФЗжзгЪНЮЊC2H6OЃЌ
ЙЪД№АИЮЊЃК ЃЛC2H6OЃЛ
ЃЛC2H6OЃЛ
ЃЈ2ЃЉCЁњDзЊЛЏЕФЗДгІЗНГЬЪНЮЊЃКCH3CH2OH
CH2=CH2+H2OЃЌ
B+FЁњGзЊЛЏЕФЗНГЬЪНЮЊЃКn +nHOCH2CH2OH
+nHOCH2CH2OH
 +ЃЈ2n-1ЃЉH2OЃЌ
+ЃЈ2n-1ЃЉH2OЃЌ
ЙЪД№АИЮЊЃКCH3CH2OH
CH2=CH2+H2OЃЛ
n +nHOCH2CH2OH
+nHOCH2CH2OH
 +ЃЈ2n-1ЃЉH2OЃЛ
+ЃЈ2n-1ЃЉH2OЃЛ
ЃЈ3ЃЉDЁњEЪєгкМгГЩЗДгІЃЌEЁњFЪєгкШЁДњЗДгІЃЌ
ЙЪД№АИЮЊЃКМгГЩЗДгІЃЛШЁДњЗДгІЃЛ
ЃЈ4ЃЉCЮЊCH3CH2OHЃЌFЮЊHOCH2CH2OHЃЌЖўепКЌгаЕФєЧЛљЪ§ФПВЛЭЌЃЌВЛЪЧЭЌЯЕЮяЃЛЖўепЗжзгЪНВЛЭЌЃЌВЛЪЧЭЌЗжвьЙЙЬхЃЌВЛЪЧЭЌжжЮяжЪЃЌЖМЪєгкДМРрЃЌЮЊЭЌРрЮяжЪЃЌ
ЙЪД№АИЮЊЃКЂлЃЎ
| 2ЁС8+2-10 |
| 2 |
 ЃЌAБЛЫсадИпУЬЫсМибѕЛЏЩњГЩBЃЌBгаЫсадЃЌдђBЮЊ
ЃЌAБЛЫсадИпУЬЫсМибѕЛЏЩњГЩBЃЌBгаЫсадЃЌдђBЮЊ ЃЎ
ЃЎ0.01molЕФCеєЦјЃЌжЪСПЮЊ0.46gЃЌдђЯрЖдЗжзгжЪСП=
| 0.46 |
| 0.01 |
| ||
| 0.01mol |
| ||
| 0.01mol |
| 46-12ЁС2-6 |
| 16 |
 ЗЂЩњЫѕОлЗДгІЩњГЩGЃЌGЮЊ
ЗЂЩњЫѕОлЗДгІЩњГЩGЃЌGЮЊ ЃЌ
ЃЌЃЈ1ЃЉAЮЊ
 ЃЌЪєгкЗМЯуЬўЭЌЗжвьЙЙЬхЕФНсЙЙМђЪНЮЊ
ЃЌЪєгкЗМЯуЬўЭЌЗжвьЙЙЬхЕФНсЙЙМђЪНЮЊ ЃЌгЩЩЯЪіЗжЮіПЩжЊCЕФЗжзгЪНЮЊC2H6OЃЌ
ЃЌгЩЩЯЪіЗжЮіПЩжЊCЕФЗжзгЪНЮЊC2H6OЃЌЙЪД№АИЮЊЃК
 ЃЛC2H6OЃЛ
ЃЛC2H6OЃЛЃЈ2ЃЉCЁњDзЊЛЏЕФЗДгІЗНГЬЪНЮЊЃКCH3CH2OH
| ХЈСђЫс |
| 170Ёц |
B+FЁњGзЊЛЏЕФЗНГЬЪНЮЊЃКn
 +nHOCH2CH2OH
+nHOCH2CH2OH| ХЈСђЫс |
| Ёї |
 +ЃЈ2n-1ЃЉH2OЃЌ
+ЃЈ2n-1ЃЉH2OЃЌЙЪД№АИЮЊЃКCH3CH2OH
| ХЈСђЫс |
| 170Ёц |
n
 +nHOCH2CH2OH
+nHOCH2CH2OH| ХЈСђЫс |
| Ёї |
 +ЃЈ2n-1ЃЉH2OЃЛ
+ЃЈ2n-1ЃЉH2OЃЛЃЈ3ЃЉDЁњEЪєгкМгГЩЗДгІЃЌEЁњFЪєгкШЁДњЗДгІЃЌ
ЙЪД№АИЮЊЃКМгГЩЗДгІЃЛШЁДњЗДгІЃЛ
ЃЈ4ЃЉCЮЊCH3CH2OHЃЌFЮЊHOCH2CH2OHЃЌЖўепКЌгаЕФєЧЛљЪ§ФПВЛЭЌЃЌВЛЪЧЭЌЯЕЮяЃЛЖўепЗжзгЪНВЛЭЌЃЌВЛЪЧЭЌЗжвьЙЙЬхЃЌВЛЪЧЭЌжжЮяжЪЃЌЖМЪєгкДМРрЃЌЮЊЭЌРрЮяжЪЃЌ
ЙЪД№АИЮЊЃКЂлЃЎ
ЕуЦРЃКБОЬтПМВщгаЛњЮяЕФЭЦЖЯгыКЯГЩЃЌМЦЫуШЗЖЈAЁЂCЕФНсЙЙЪЧНтЬтЙиМќЃЌНсЙЙзЊЛЏЙиЯЕИљОнЙйФмЭХЕФаджЪгызЊЛЏНјааЭЦЖЯЃЌФбЖШжаЕШЃЌзЂвтеЦЮеЙйФмЭХЕФаджЪгызЊЛЏЃЎ

СЗЯАВсЯЕСаД№АИ
ЯрЙиЬтФП
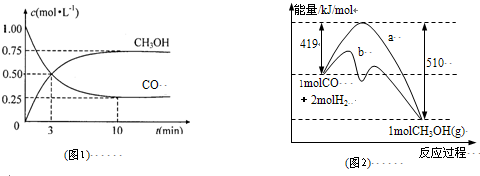
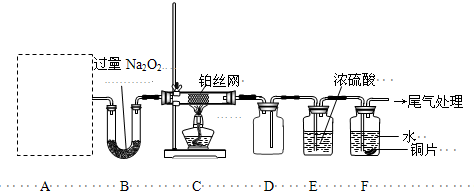
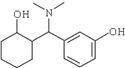 ЃЉЪЧвЛжжеђЭДКЭТщзэвЉЮяЃЌПЩгЩЛЏКЯЮяBЃЈ
ЃЉЪЧвЛжжеђЭДКЭТщзэвЉЮяЃЌПЩгЩЛЏКЯЮяBЃЈ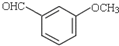 ЃЉЭЈЙ§вдЯТТЗЯпКЯГЩЕУЕНЃЎ
ЃЉЭЈЙ§вдЯТТЗЯпКЯГЩЕУЕНЃЎ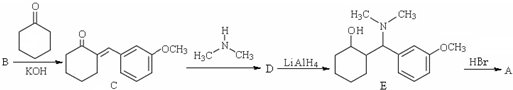
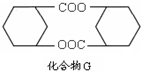 ЃЉЕФКЯГЩТЗЯпСїГЬЭМЃЈЮоЛњЪдМСШЮбЁЃЉЃЎ
ЃЉЕФКЯГЩТЗЯпСїГЬЭМЃЈЮоЛњЪдМСШЮбЁЃЉЃЎ