��Ŀ����
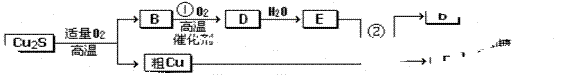
��8�֣���ͭ�����ɷ�ΪCu2S��ͨ�����Ŀ�����ұ������ͭ������һϵ�з�Ӧ�ɵõ�B��D��E��GΪש��ɫ������
��ش��������⣺
��1����ͭ��Cu2S��ͨ �����Ŀ���ұ������ͭ�Ļ�ѧ����ʽ__________________________
�����Ŀ���ұ������ͭ�Ļ�ѧ����ʽ__________________________
______����������Ϊ____________________��
��2��E��Ũ��Һ��Cu������Ӧ�ڵĻ�ѧ����ʽ�� ��
��3�����õ����ᴿ�֣��ڸõ�ⷴӦ������������ ���������Һ�� ��
��4����Ȼ���е��������������FeS2��������������ʱ�Ỻ���������з�Ӧ������ͭ�Է�Ӧ��14CuSO4+ 5FeS2 +12H2O==7Cu2S+5FeSO 4+12H2SO4
4+12H2SO4
����������ͱ���ԭ�����������Ϊ_________________________��

��ش��������⣺
��1����ͭ��Cu2S��ͨ
 �����Ŀ���ұ������ͭ�Ļ�ѧ����ʽ__________________________
�����Ŀ���ұ������ͭ�Ļ�ѧ����ʽ________________________________����������Ϊ____________________��
��2��E��Ũ��Һ��Cu������Ӧ�ڵĻ�ѧ����ʽ�� ��
��3�����õ����ᴿ�֣��ڸõ�ⷴӦ������������ ���������Һ�� ��
��4����Ȼ���е��������������FeS2��������������ʱ�Ỻ���������з�Ӧ������ͭ�Է�Ӧ��14CuSO4+ 5FeS2 +12H2O==7Cu2S+5FeSO
 4+12H2SO4
4+12H2SO4 ����������ͱ���ԭ�����������Ϊ_________________________��
(1) Cu2S+O2�������� 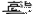 2Cu+SO2 ��������O2�� Cu2S ��
2Cu+SO2 ��������O2�� Cu2S ��
(2) Cu+2H2SO4(Ũ)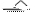 CuSO4+SO2��+2H2O ��
CuSO4+SO2��+2H2O ��
(3) ��ͭ ������ͭ��Cu2+������Һ ��
(4) 3��7
 2Cu+SO2 ��������O2�� Cu2S ��
2Cu+SO2 ��������O2�� Cu2S �� (2) Cu+2H2SO4(Ũ)
 CuSO4+SO2��+2H2O ��
CuSO4+SO2��+2H2O �� (3) ��ͭ ������ͭ��Cu2+������Һ ��
(4) 3��7
��

��ϰ��ϵ�д�
�����Ŀ
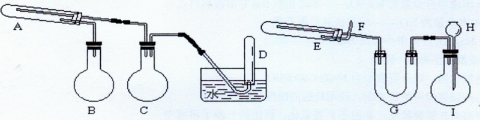
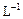 �����У�����ҺC��
�����У�����ҺC��
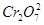 ����ˮ�����������[
����ˮ�����������[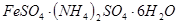 ]��������Ӧ����Ԫ�غ�Ԫ��
]��������Ӧ����Ԫ�غ�Ԫ�� ��ȫת��Ϊ�������ó��������õ�
��ȫת��Ϊ�������ó��������õ� �������Ǵ��������е�ʵ����ģ����������������
�������Ǵ��������е�ʵ����ģ����������������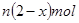
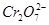 �����ʵ���Ϊ
�����ʵ���Ϊ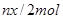
 ��ת�Ƶĵ�����Ϊ
��ת�Ƶĵ�����Ϊ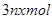
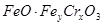 ����
����