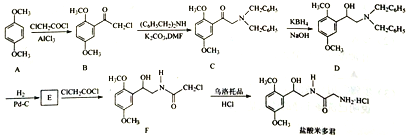
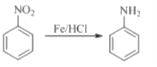
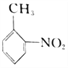
��Ŀ����
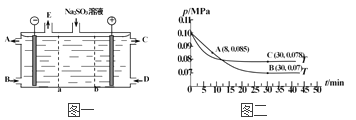
����Ŀ������ʱ����2molA��2molB����Ͷ��̶��ݻ�Ϊ2L�ܱ������з�����Ӧ��2A��g��+ B��g��![]() xC��g��+D��s����10sʱ�����A�����ʵ���Ϊ1.7mol��C�ķ�Ӧ����Ϊ0.0225mol��L��1��s��1��40sʱ��Ӧǡ�ô���ƽ��״̬����ʱB��ת����Ϊ20%������д���пհף�
xC��g��+D��s����10sʱ�����A�����ʵ���Ϊ1.7mol��C�ķ�Ӧ����Ϊ0.0225mol��L��1��s��1��40sʱ��Ӧǡ�ô���ƽ��״̬����ʱB��ת����Ϊ20%������д���пհף�
��1��x = _________________
��2���ӷ�Ӧ��ʼ��10s��B��ƽ����Ӧ����Ϊ______________
��3��ƽ��ʱ������B���������Ϊ___________________
��4�����¶��´˷�Ӧ��ƽ�ⳣ������ʽΪ_______________
��5�����и����ܱ�ʾ�÷�Ӧ�ﵽƽ��״̬��_____________
A������A�����ʵ���������D�����ʵ���֮��Ϊ2��1
B��������A��B�����ʵ��� n(A)��n(B) = 2��1
C�������ƽ����Է����������ٱ仯
D��ѹǿ���ٱ仯
E����������ʵ������ٱ仯
���𰸡� 3 0.0075 mol(L��s)��1 40% ![]() C
C
��������(1)10s����n(A)=2mol-1.7mol=0.3mol����n(C)=0.0225molL-1s-1��10s��2L=0.45mol�����ʵ����仯��֮�ȵ��ڻ�ѧ������֮�ȣ���2��x=0.3mol��0.45mol�����x=3���ʴ�Ϊ��3��
(2)��������֮�ȵ��ڻ�ѧ������֮�ȣ���v(B)= ![]() v(C)=
v(C)= ![]() ��0.0225molL-1s-1=0.0075molL-1s-1���ʴ�Ϊ��0.0075molL-1s-1��
��0.0225molL-1s-1=0.0075molL-1s-1���ʴ�Ϊ��0.0075molL-1s-1��
(3)40sʱ��Ӧǡ�ô���ƽ��״̬����ʱB��ת����Ϊ20%����Ӧ��BΪ2mol��20%=0.4mol��ƽ��ʱBΪ2mol-0.4mol=1.6mol����Ӧǰ������������ʵ������䣬��B���������Ϊ![]() ��100%=40%���ʴ�Ϊ��40%��
��100%=40%���ʴ�Ϊ��40%��
(4)2A(g)+B(g) ![]() xC (g)+D(s)�Ļ�ѧƽ�ⳣ������ʽK=
xC (g)+D(s)�Ļ�ѧƽ�ⳣ������ʽK=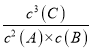 ���ʴ�Ϊ��
���ʴ�Ϊ�� 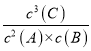 ��
��
(5)A������A�����ʵ���������D�����ʵ���֮��ʼ��Ϊ2��1������˵������ƽ�⣬��A����B��ƽ��ʱ��������ʵ���֮�Ȳ�һ�����ڻ�ѧ������֮�ȣ���A��B��ʼ���ʵ���Ϊ1��1�����߰�2��1��Ӧ��ƽ��ʱ�������ʵ���֮�Ȳ�����Ϊ2��1����B����C����Ӧǰ�����������ʵ������䣬�淴Ӧ������������������С�������ƽ����Է����������ٱ仯��˵������������������䣬��Ӧ����ƽ�⣬��C��ȷ��D��x=3����Ӧǰ�����������ʵ������䣬���º����£�ѹǿʼ�ղ��䣬����˵���ﵽ��ƽ��״̬����D����E��x=3���÷�Ӧ������������ʵ�������ķ�Ӧ�����ʵ���ʼ�ղ��䣬����˵���ﵽ��ƽ��״̬����E����ѡC��