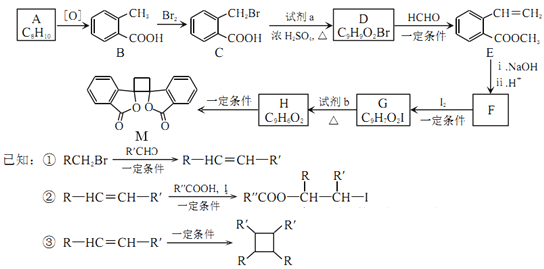
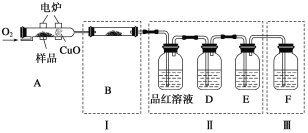
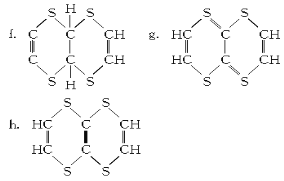
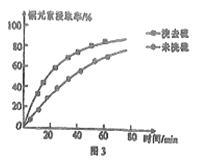
��Ŀ����
����Ŀ����֪������(H2SeO3)Ϊ��Ԫ���ᣬ�����£���ijŨ�ȵ���������Һ����μ���һ��Ũ�ȵ�NaOH��Һ��������Һ��H2SeO3��HSeO3-��SeO32-�����������ʵ�����������ҺpH �Ĺ�ϵ��ͼ��ʾ������˵������ȷ����

A. �����£�������ĵ���ƽ�ⳣ��K2=10-4.2
B. pH=l.2����Һ�У�c(Na+)+c(H+)=c(OH-)+c(H2SeO3)
C. ����ͬ���ʵ���NaHSeO3��Na2SeO3 ������ȫ����ˮ�����pHΪ4.2�Ļ��Һ
D. ��pH=1.2����Һ�еμ�NaOH��Һ��pH=4.2�Ĺ�����ˮ�ĵ���̶�һֱ����
���𰸡�C
��������A:���ݵ��뷴Ӧ����ʽ��H2SeO3=H++HSeO3-��HSeO3-= H++ SeO32-֪K2=c(H+).c(SeO32-) /c(HSeO3-),��PH=4.2ʱ���ﵽƽ�����ԡ���ʱc(HSeO3-)=c(SeO32-)
˵��K2=10-4.2��A��ȷ��B.pH=l.2����Һ��H2SeO3��HSeO3-��Ũ����ȣ����ݵ���غ��ϵ֪��c(Na+)+c(H+)=c(OH-)+c(H2SeO3)����ȷ�ģ���B�ԣ�C.����ͬ���ʵ���NaHSeO3��Na2SeO3 ������ȫ����ˮ�������pHΪ4.2�Ļ��Һ��Na2SeO3ǿ�������Σ�ˮ���Լ��ԣ�NaHSeO3ˮ��ﵽƽ��ʱPH=4.2��������������PHһ������4,2.��C����D.��pH=12����Һ�еμ�NaOH��Һ��pH=4.2�Ĺ�������Ϊ�к�����Һ�е������ӣ��ٽ���ˮ�ĵ��룬��ˮ�ĵ���̶�һֱ����D��ȷ��

 ��У����ϵ�д�
��У����ϵ�д�