��Ŀ����
�����ǹ㷺ʹ�õķ��ϣ������йز��ϣ��ش����⣮
(1)�������ũ�峣�õ�һ���̬���ʣ�
��������������������__________��
��ʵ����ij����立����е�Ԫ�ص���������Ϊ20���������Ʒ�п��ܻ���_____________��
[����]
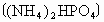
(2)�����������ճ�������˾�ռ��ߵ����������緢���Ĺ����У������еĵ���������ֱ�ӻ��ϣ�����ij����ų�������Ϊ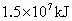 ����֪
����֪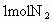 ��
��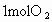 ����ʱҪ����181kJ����������ʱ��1/1000������������һ��Ӧ��
����ʱҪ����181kJ����������ʱ��1/1000������������һ��Ӧ��
�ٴ˴�������������NO�����ʵ�����_____mol.
�ڴ˴�����������൱�ڸ�����ʩ����_______ǧ�˵����ػ��ʡ����ػ�ѧʽ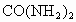 ��.
��.
������
|
(1) ��21������A(��ʾ����������������С��20��)(2) ��һ��������������NO��������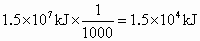
���� NO�����ʵ���Ϊ
���������൱������ 41.5mol����Ϊ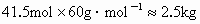 |

 ��У����ϵ�д�
��У����ϵ�д���11 �֣���ѧ��һֱ�����о����¡���ѹ�¡��˹��̵������·���������ʵ�鱨�����ڳ��¡���ѹ�����������£�N2�ڴ�������������Fe2O3��TiO2��������ˮ������Ӧ�����ɵ���Ҫ����ΪNH3����Ӧ�Ļ�ѧ����ʽ���£�N2(g)+ 3H2O(l)  2NH3(g)+ O2(g)���ش��������⣺
2NH3(g)+ O2(g)���ش��������⣺
��1����һ���о�NH3���������¶ȵĹ�ϵ������ʵ�����ݼ��±������ա�N2ѹ��1.0��105 Pa����Ӧʱ��3 h������÷�Ӧ������ӦΪ ��Ӧ������ȡ����ȡ���
|
T/K |
303 |
313 |
323 |
|
NH3������/��10-6 mol�� |
4.8 |
5.9 |
6.0 |
��2����Ŀǰ�㷺ʹ�õĹ�ҵ�ϳɰ�������ȣ��÷����й̵���Ӧ�������������������䷴Ӧ����������NH3�������Ľ��飺���� ��
��3���ϳɰ���ҵ��ԭ����N2�ɴӿ����з���õ���H2���ü����ڸ�������ˮ������Ӧ�Ƶá������ڸ�������ˮ������Ӧ��Ӧ����ʽΪ��CH4(g)��H2O(g)��CO(g)��3H2(g)���������ʵ�ȼ������
�����£�
H2(g) ����H =��285.8 kJ・mol��1��
CO(g) �� ��H =��283.0 kJ・mol��1��
CH4(g) ����H =��890.3 kJ・mol��1 ��
��֪1mol H2O(g)ת��Ϊ1mol H2O(l)ʱ�ų�44.0 kJ������д��CH4��H2O�ڸ����·�Ӧ���Ȼ�ѧ����ʽ__________________________________��
��4����������Ѱ���ʺϵĴ����͵缫���ϣ���N2��H2Ϊ�缫��Ӧ���HCl����NH4ClΪ�������Һ�Ƴ�����ȼ�ϵ�أ���д���õ缫��������Ӧʽ
��5�����ɵ�NH3���������̬���ʣ���(NH4)2SO4��NH4Cl����Щ������ �ԣ�ԭ���ǣ������ӷ���ʽ��ʾ��___________________________��ʹ��ʱ������________________���ʺ�ʩ��
 NH3H2O+H+
NH3H2O+H+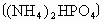
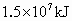 ����֪
����֪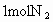 ��
�� ����ʱҪ����181kJ����������ʱ��1/1000������������һ��Ӧ��
����ʱҪ����181kJ����������ʱ��1/1000������������һ��Ӧ��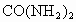 ��.
��.