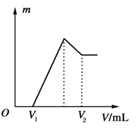
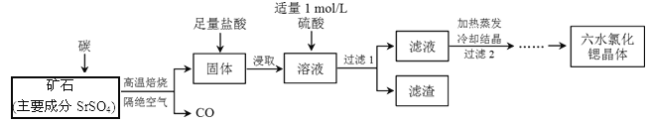
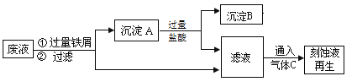
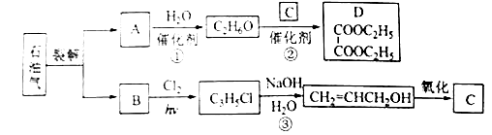
��Ŀ����
����Ŀ��ˮ��������(DO)�Ǻ���ˮ���Ծ�������һ��ָ�꣬ͨ����ÿ��ˮ���ܽ������ӵ�������ʾ����λmg/L���ҹ����ر�ˮ�������������涨����������ˮԴ��DO���ܵ���5mg/L��ij��ѧС��ͬѧ���������װ��(�г�װ����)���ⶨij��ˮ��DO��
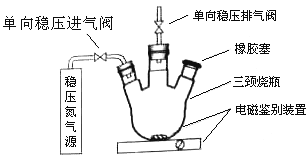
1���ⶨԭ����
��������£�O2��Mn2+����ΪMnO(OH)2:��2Mn2++O2+4OH-=2 MnO(OH)2�������������£�MnO(OH)2��I-����ΪI2:��MnO(OH)2+I-+H+��Mn2++I2+H2O(δ��ƽ)����Na2S2O3����Һ�ζ����ɵ�I2����2S2O32-+I2=S4O62-+2I-
2���ⶨ����
a����װװ�ã����������ԣ���N2�ž�������ֹͣ��N2��
b������ƿ�м���200mlˮ��
c������ƿ������Ѹ�ټ���1mlMnSO4������Һ(����)2ml����KI������Һ(����)������������������Ӧ����ȫ��
d���貢����ƿ�м���2ml����������Һ����Ӧ����ȫ����ҺΪ���Ի������ԡ�
e������ƿ��ȡ��40��00ml��Һ���Ե�����ָʾ������0��001000mol/L Na2S2O3��Һ���еζ�����¼���ݡ�
f������
g����������(����������ˮ���е�������ͼ����Լ���ˮ������ı仯)��
�ش��������⣺
��1����������������Һʱ����ȥ�����ܼ�ˮ�����ļ���Ϊ__________��
��2��������������ˮ�����й��Լ�Ӧѡ�������Ϊ__________��
���ζ�����ע��������Ͳ
��3�������������__________��
��4����ƽ��Ӧ���ķ���ʽ���仯ѧ����������Ϊ__________��
��5������fΪ__________��
��6������e�дﵽ�ζ��յ�ı�־Ϊ__________����ij�εζ�����Na2S2O3��Һ4.50ml��ˮ����DO=__________mg/L(����һλС��)����Ϊ����ˮԴ���˴β��DO�Ƿ��꣺__________(���ǻ��)
��7������d�м���������Һ��Ӧ������ҺpH���ͣ��ζ�ʱ��������Ե���д������������ԭ��(�����ӷ���ʽ��ʾ������д��2��)__________��
���𰸡�
��1�����ܼ�ˮ��к���ȴ
��2����
��3��ʹ��Һ��Ͼ��ȣ�������ɷ�Ӧ
��4��1��2��4��1��1��3
��5���ظ�����e�IJ���2-3��
��6����Һ��ɫ��ȥ(������ڲ���ɫ) 9.0 ��
��7��2H++S2O32-=S��+SO2��+H2O��SO2+I2+2H2O=4H++SO42-+2I-��4H++4I-+O2=2I2+2H2O(�����2��)
��������
�����������1��������ˮ�е��ܽ�������¶����߶���С�����ܼ�ˮ��п��Գ�ȥ�����ܼ�ˮ�������ʴ�Ϊ�����ܼ�ˮ��к���ȴ��
��2��������������ˮ�����й��Լ�Ӧѡ��ע��������ѡ����
��3���������ʹ��Һ��Ͼ��ȣ��ӿ췴Ӧ�����ʣ��ʴ�Ϊ��ʹ��Һ��Ͼ��ȣ�������ɷ�Ӧ��
��4�����ݻ��ϼ������غ㣬��Ӧ����ƽ��MnO(OH)2+2I-+4H+=Mn2++I2+3H2O���ʴ�Ϊ��1��2��4��1��1��3��
��5���ζ�����һ����Ҫ�ظ�����2-3�Σ��Ա��Сʵ������˲���fΪ�ظ�����e�IJ���2-3�Σ��ʴ�Ϊ���ظ�����e�IJ���2-3�Σ�
��6�������ӱ�����Ϊ�ⵥ�ʺ���Na2S2O3��Һ�ζ����ԭΪ�����ӣ���˵ζ���������Һ����ɫ��ʧ��n(Na2S2O3)= 0.001000mol/L��0.0045L=4.5��10-6mol�����ݷ�Ӧ�٢ڢ���O2~2 MnO(OH)2��~ 2I2~4S2O32-��n(O2)=![]() n(Na2S2O3)=1.125��10-6mol���ú�ˮ��DO=
n(Na2S2O3)=1.125��10-6mol���ú�ˮ��DO=![]() ��1.125��10-6��32=9��10-3g/L=9.0 mg/L��5 mg/L����꣬�ʴ�Ϊ����Һ��ɫ��ȥ(������ڲ���ɫ)��9.0���ǣ�
��1.125��10-6��32=9��10-3g/L=9.0 mg/L��5 mg/L����꣬�ʴ�Ϊ����Һ��ɫ��ȥ(������ڲ���ɫ)��9.0���ǣ�
��7����������������������·����绯��Ӧ�����ɵĶ�������Ҳ�ܹ������ɵĵ�������ͬʱ�����е�����Ҳ�ܹ�����������������Ӧ�����ӷ���ʽ�ֱ�Ϊ��2H++S2O32-=S��+SO2��+H2O��SO2+I2+2H2O=4H++SO42-+2I-��4H++4I-+O2=2I2+2H2O���ʴ�Ϊ��2H++S2O32-=S��+SO2��+H2O��SO2+I2+2H2O=4H++SO42-+2I-��4H++4I-+O2=2I2+2H2O��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�