��Ŀ����
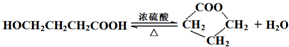
ij�����廯����A�ķ����к���C��H��O��N����Ԫ�أ���ͬ״���£����������ܶ�Ϊ�����ܶȵ�68.5�������Ա�Ϊԭ�Ϻϳ�A���������Ƶ�F��һ��Ⱦ���м��壩��ת����ϵ���£�һЩ����Ҫ���Z��ȥ����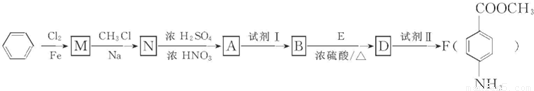
��֪��
��ش��������⣺
��1��д�� A�Ľṹ��ʽ______��
��2��N��A�ķ�Ӧ������______��
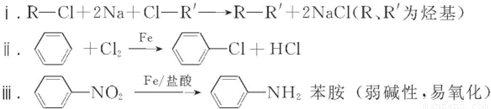
��3������ת�����Լ�I���Լ���ֱ��ǣ��Լ�I______���Լ���______��ѡ����ĸ����
a��KMnO4��H+�� b��Fe/���� c��NaOH��Һ
��4���ú˴Ź���������֤��������E�к���______�ִ��ڲ�ͬ��ѧ�������⣮
��5��д��ͬʱ��������Ҫ�������D��ͬ���칹��Ľṹ��ʽ��
�����ڷ����廯�����������������Ϊ��λ��ȡ����������һ��ȡ������������
�ڷ����к���
 �ṹ��______
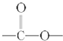
�ṹ��______��6����һ��D��ͬ���칹��w��������������ˮ��ɵõ�һ������FeCl3��Һ������ɫ��Ӧ�IJ��д��w������������ˮ��Ļ�ѧ����ʽ��______��
��7��F��ˮ�ⷴӦ���£�
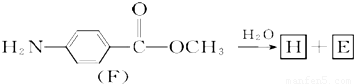 ������H��һ�������¾����۷�Ӧ���Ƶø߷�����ά���㷺����ͨѶ�����������������д�������۷�Ӧ�Ļ�ѧ����ʽ��
������H��һ�������¾����۷�Ӧ���Ƶø߷�����ά���㷺����ͨѶ�����������������д�������۷�Ӧ�Ļ�ѧ����ʽ��______��
���𰸡��������������Ϣ��֪NӦΪ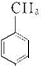 ����Ũ���������������ᷢ��ȡ����Ӧ������
����Ũ���������������ᷢ��ȡ����Ӧ������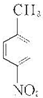 ����AΪ
����AΪ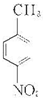 ����Է�������Ϊ137���������ܶ�Ϊ�����ܶȵ�68.5����A�����Ը��������������BΪ
����Է�������Ϊ137���������ܶ�Ϊ�����ܶȵ�68.5����A�����Ը��������������BΪ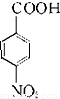 ����״���Ũ���������·�Ӧ����DΪ
����״���Ũ���������·�Ӧ����DΪ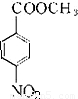 ��Ȼ������ԭ��Ӧ����F������л���Ľṹ�����ʽ����⣮
��Ȼ������ԭ��Ӧ����F������л���Ľṹ�����ʽ����⣮
����⣺�������Ϣ��֪NӦΪ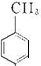 ����Ũ���������������ᷢ��ȡ����Ӧ������
����Ũ���������������ᷢ��ȡ����Ӧ������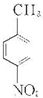 ����AΪ
����AΪ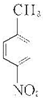 ����Է�������Ϊ137���������ܶ�Ϊ�����ܶȵ�68.5����A�����Ը��������������BΪ
����Է�������Ϊ137���������ܶ�Ϊ�����ܶȵ�68.5����A�����Ը��������������BΪ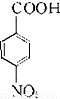 ����״���Ũ���������·�Ӧ����DΪ
����״���Ũ���������·�Ӧ����DΪ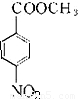 ��Ȼ������ԭ��Ӧ����F��
��Ȼ������ԭ��Ӧ����F��
��1�������Ϸ�����֪AΪ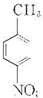 ���ʴ�Ϊ��
���ʴ�Ϊ��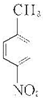 ��
��
��2��NΪ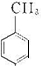 ����Ũ���������������ᷢ��ȡ����Ӧ������
����Ũ���������������ᷢ��ȡ����Ӧ������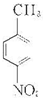 ���ʴ�Ϊ��ȡ����Ӧ��
���ʴ�Ϊ��ȡ����Ӧ��
��3���������Ϣ������Ϸ�����֪�Լ�I���Լ���ֱ���KMnO4��H+����Fe/���ᣬ�ʴ�Ϊ��a��b��
��4��EΪCH3OH���������ֲ�ͬ��Hԭ�ӣ��ʴ�Ϊ��2��
��5��DΪ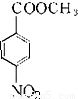 ����Ӧ�����ڷ����廯�����������������Ϊ��λ��ȡ����������һ��ȡ�������������ҷ����к���
����Ӧ�����ڷ����廯�����������������Ϊ��λ��ȡ����������һ��ȡ�������������ҷ����к���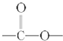 �ṹ��ͬ���칹����
�ṹ��ͬ���칹����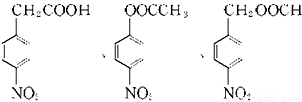 ��
��
�ʴ�Ϊ��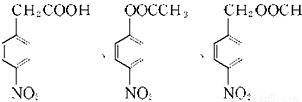 ��
��
��6��D��ͬ���칹��w��������������ˮ��ɵõ�һ������FeCl3��Һ������ɫ��Ӧ�IJ��˵�������к��з��ǻ���ӦΪ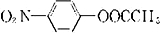 ��ˮ��ķ���ʽΪ
��ˮ��ķ���ʽΪ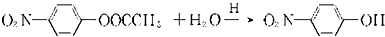 +CH3COOH��
+CH3COOH��
�ʴ�Ϊ��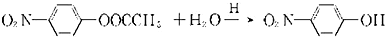 +CH3COOH��
+CH3COOH��
��7��F��ˮ�����Ϊ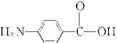 ��CH3OH��������H��һ�������¾����۷�Ӧ���Ƶø߷�����ά���㷺����ͨѶ�����������������HΪ
��CH3OH��������H��һ�������¾����۷�Ӧ���Ƶø߷�����ά���㷺����ͨѶ�����������������HΪ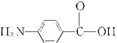 ���ɷ������۷�Ӧ������ʽΪ
���ɷ������۷�Ӧ������ʽΪ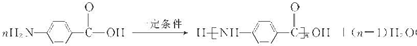 ��
��
�ʴ�Ϊ��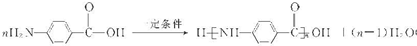 ��
��
���������⿼���л�����ƶϺͺϳɣ���Ŀ�ѶȽϴ���ע����������Ϣ��Ϊ������Ĺؼ���ע���л�������ŵ����ʺͱ仯��
 ����Ũ���������������ᷢ��ȡ����Ӧ������
����Ũ���������������ᷢ��ȡ����Ӧ������ ����AΪ
����AΪ ����Է�������Ϊ137���������ܶ�Ϊ�����ܶȵ�68.5����A�����Ը��������������BΪ
����Է�������Ϊ137���������ܶ�Ϊ�����ܶȵ�68.5����A�����Ը��������������BΪ ����״���Ũ���������·�Ӧ����DΪ
����״���Ũ���������·�Ӧ����DΪ ��Ȼ������ԭ��Ӧ����F������л���Ľṹ�����ʽ����⣮
��Ȼ������ԭ��Ӧ����F������л���Ľṹ�����ʽ����⣮����⣺�������Ϣ��֪NӦΪ
 ����Ũ���������������ᷢ��ȡ����Ӧ������
����Ũ���������������ᷢ��ȡ����Ӧ������ ����AΪ
����AΪ ����Է�������Ϊ137���������ܶ�Ϊ�����ܶȵ�68.5����A�����Ը��������������BΪ
����Է�������Ϊ137���������ܶ�Ϊ�����ܶȵ�68.5����A�����Ը��������������BΪ ����״���Ũ���������·�Ӧ����DΪ
����״���Ũ���������·�Ӧ����DΪ ��Ȼ������ԭ��Ӧ����F��
��Ȼ������ԭ��Ӧ����F����1�������Ϸ�����֪AΪ
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����2��NΪ
 ����Ũ���������������ᷢ��ȡ����Ӧ������
����Ũ���������������ᷢ��ȡ����Ӧ������ ���ʴ�Ϊ��ȡ����Ӧ��
���ʴ�Ϊ��ȡ����Ӧ����3���������Ϣ������Ϸ�����֪�Լ�I���Լ���ֱ���KMnO4��H+����Fe/���ᣬ�ʴ�Ϊ��a��b��
��4��EΪCH3OH���������ֲ�ͬ��Hԭ�ӣ��ʴ�Ϊ��2��
��5��DΪ
 ����Ӧ�����ڷ����廯�����������������Ϊ��λ��ȡ����������һ��ȡ�������������ҷ����к���
����Ӧ�����ڷ����廯�����������������Ϊ��λ��ȡ����������һ��ȡ�������������ҷ����к��� �ṹ��ͬ���칹����
�ṹ��ͬ���칹���� ��
���ʴ�Ϊ��
 ��
����6��D��ͬ���칹��w��������������ˮ��ɵõ�һ������FeCl3��Һ������ɫ��Ӧ�IJ��˵�������к��з��ǻ���ӦΪ
 ��ˮ��ķ���ʽΪ
��ˮ��ķ���ʽΪ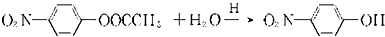 +CH3COOH��
+CH3COOH���ʴ�Ϊ��
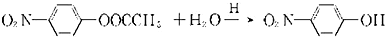 +CH3COOH��
+CH3COOH����7��F��ˮ�����Ϊ
 ��CH3OH��������H��һ�������¾����۷�Ӧ���Ƶø߷�����ά���㷺����ͨѶ�����������������HΪ
��CH3OH��������H��һ�������¾����۷�Ӧ���Ƶø߷�����ά���㷺����ͨѶ�����������������HΪ ���ɷ������۷�Ӧ������ʽΪ
���ɷ������۷�Ӧ������ʽΪ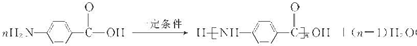 ��
���ʴ�Ϊ��
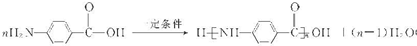 ��
�����������⿼���л�����ƶϺͺϳɣ���Ŀ�ѶȽϴ���ע����������Ϣ��Ϊ������Ĺؼ���ע���л�������ŵ����ʺͱ仯��

��ϰ��ϵ�д�
�����Ŀ



 �ṹ��
�ṹ��










 �ṹ��
�ṹ��
 +CH3COOH
+CH3COOH ������H��һ�������¾����۷�Ӧ���Ƶø߷�����ά���㷺����ͨѶ�����������������д�������۷�Ӧ�Ļ�ѧ����ʽ��
������H��һ�������¾����۷�Ӧ���Ƶø߷�����ά���㷺����ͨѶ�����������������д�������۷�Ӧ�Ļ�ѧ����ʽ��
 ������RΪ������������A��һ������������ͼ��ʾ��ת����ϵ����֪E�������ܶ�����ͬ������H2�ܶȵ�74����������ɷ���CaHbO2��
������RΪ������������A��һ������������ͼ��ʾ��ת����ϵ����֪E�������ܶ�����ͬ������H2�ܶȵ�74����������ɷ���CaHbO2��