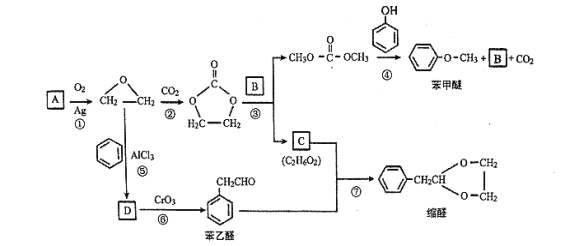
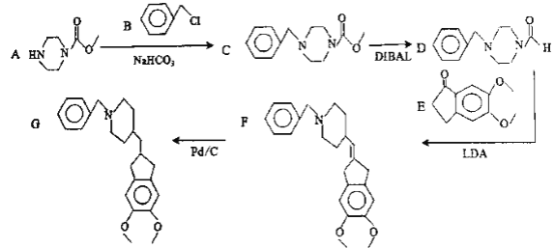
��Ŀ����
����Ŀ��ƫ��������N2O4�dz��õĻ���ƽ��������߷������»�ѧ��Ӧ��(CH3)2NNH2(l)+2N2O4(l)��2CO2(g)+3N2(g)+4H2O(g) (��)
��1����Ӧ(��)����������_________��
��2������к��г����ֺ���ɫ���壬����Ϊ��N2O4(g)![]() 2NO2(g)(��)�����¶�����ʱ��������ɫ�����Ӧ(��)Ϊ____________(����ȡ����ȡ�)��Ӧ��
2NO2(g)(��)�����¶�����ʱ��������ɫ�����Ӧ(��)Ϊ____________(����ȡ����ȡ�)��Ӧ��
��3��550��ʱ����2.0molSO2��1.0molO2����5L�ܱ������У���Ӧ��ƽ������д�ʩ����ʹn(SO3)/n(SO2)�������_____________��
A.�����¶�
B.����He(g)��ʹ��ϵ��ѹǿ����
C.�ٳ���2.0molSO2��1.0molO2
���𰸡�N2O4 ���� C
��������
(1)��֪�ڷ�Ӧ (CH3)2NNH2(l)+2N2O4(l)��2CO2(g)+3N2(g)+4H2O(g)�У�CԪ�ش�-2�����ߵ�+4����ƫ�������е�NԪ�ش�-2�����ߵ�0�ۣ�����(CH3)2NNH2����ԭ������N2O4�е�NԪ�ش�+4�۽��͵�0������N2O4����������
(2)���ڷ�ӦN2O4(g)![]() 2NO2(g)�����¶�����ʱ��������ɫ���˵����Ӧ����NO2�������ˣ��������������ƶ�����÷�ӦΪ���ȷ�Ӧ��
2NO2(g)�����¶�����ʱ��������ɫ���˵����Ӧ����NO2�������ˣ��������������ƶ�����÷�ӦΪ���ȷ�Ӧ��
(3)���ݷ�Ӧ����ʽ2SO2+ O2 ![]() 2SO3 ��H��0���������£�A�������¶ȣ�ƽ�������ƶ���ʹn(SO3)��С��n(SO2)������n(SO3)/n(SO2)�Ǽ�С�ģ���A����B������He(g)��ʹ��ϵ��ѹǿ����������Ӧ��ϵ�ķ�ѹ���䣬����ƽ�ⲻ�ƶ�����n(SO3)/n(SO2)�Dz������B����C���ٳ���2.0 mol SO2��1.0 mol O2���൱�ڼ�ѹ����ƽ�������ƶ���ʹn(SO3) ������n(SO2) ��С����n(SO3)/n(SO2)������ģ���C��ȷ��������ȷ��ΪC��
2SO3 ��H��0���������£�A�������¶ȣ�ƽ�������ƶ���ʹn(SO3)��С��n(SO2)������n(SO3)/n(SO2)�Ǽ�С�ģ���A����B������He(g)��ʹ��ϵ��ѹǿ����������Ӧ��ϵ�ķ�ѹ���䣬����ƽ�ⲻ�ƶ�����n(SO3)/n(SO2)�Dz������B����C���ٳ���2.0 mol SO2��1.0 mol O2���൱�ڼ�ѹ����ƽ�������ƶ���ʹn(SO3) ������n(SO2) ��С����n(SO3)/n(SO2)������ģ���C��ȷ��������ȷ��ΪC��

����Ŀ������������������Ⱦ�������أ�ԭ��֮һ�ǻ�����β���к���NO��NO2��CO�����壬�������糧�ͷų�������NOx��SO2��CO2������Ҳ����ԭ�����ڶ����е�һЩ���������һ�����о���
��1���� CH4����ԭ��������������������������Ⱦ��
��֪����CH4(g) + 4NO2(g) = 4NO(g) + CO2(g) + 2H2O(g) ��H = - 574 kJ/mol
��CH4(g) + 4NO(g) = 2N2(g) + CO2(g) + 2H2O(g) ��H = - 1160 kJ/mol
��H2O(g) = H2O(l) ��H = - 44.0 kJ/mol
д�� CH4(g)�� NO2(g)��Ӧ���� N2(g)��CO2(g)�� H2O(l)���Ȼ�ѧ����ʽ�� _____________________________________________________________��
��2������β���к���CO��NO2���ж���������������װβ������װ������ʹ�ж�����ת��Ϊ�����塣4CO(g)��2NO2(g) ![]() 4CO2(g)��N2(g) ��H��-1200 kJ��mol-1
4CO2(g)��N2(g) ��H��-1200 kJ��mol-1
���ڸ÷�Ӧ���¶Ȳ�ͬ��T2��T1��������������ͬʱ������ͼ����ȷ����________________������ţ���

��3���û���̿��ԭ��Ҳ���Դ����������ij�о�С����ij�ܱ���������һ�����Ļ���̿��NO��������ӦC(s)+2NO(g) ![]() N2(g)+CO2(g) ��H=a kJ/mol
N2(g)+CO2(g) ��H=a kJ/mol
��T1��ʱ����Ӧ���е���ͬʱ���ø����ʵ���Ũ�����£�
ʱ��/min Ũ��/(mol/L) | 0 | 10 | 20 | 30 | 40 | 50 |
NO | 1.0 | 0.58 | 0.40 | 0.40 | 0.48 | 0.48 |
N2 | 0 | 0.21 | 0.30 | 0.30 | 0.36 | 0.36 |
CO2 | 0 | 0.21 | 0.30 | 0.30 | 0.36 | 0.36 |
�ٸ���ͼ�����ݷ���T1��ʱ���÷�Ӧ��0-20min��ƽ����Ӧ����v��NO��=_____________________������÷�Ӧ��ƽ�ⳣ��K=____________________��
��30min��ֻ�ı�ijһ�����������ϱ��������жϸı������������_________������ĸ���ţ���
A��ͨ��һ������CO2 B��������ʵĴ���
C���ʵ���С��������� D��ͨ��һ������NO
E������һ�����Ļ���̿
����30min�������¶���T2�����ﵽƽ��ʱ��������NO��N2��CO2��Ũ��֮��Ϊ2:1:1����ﵽ��ƽ��ʱNO��ת����____________��������������������������a________0������>������<������
��4���¶�T1��T2ʱ���ֱ�0.50 mol CH4��1.2mol NO2����1 L���ܱ������з�����Ӧ��CH4(g)��2NO2(g)![]() N2(g)��CO2(g)��2H2O(g) ��H=bkJ/mol��
N2(g)��CO2(g)��2H2O(g) ��H=bkJ/mol��
����й��������±���
�¶� | ʱ��/min ���ʵ��� | 0 | 10 | 20 | 40 | 50 |
T1 | n��CH4��/mol | 0.50 | 0.35 | 0.25 | 0.10 | 0.10 |
T2 | n��CH4��/mol | 0.50 | 0.30 | 0.18 | x | 0.15 |
����˵����ȷ����__________________��
A��T1��T2����b��0
B�����¶�ΪT2����Ӧ���е�40 minʱ�� x��0.15
C���¶�ΪT2ʱ������ƽ�����������ٳ���0.50 mol CH4��1.2mol NO2�����´ﵽƽ��ʱ��n��N2��<0.70mol
D���¶�ΪT1ʱ������ʼʱ�������г���0.50 mol CH4(g)��0.50 molNO2(g)��1.0 mol N2(g)��2.0 molCO2(g)��0.50molH2O(g)����Ӧ��ʼʱ����(��)����(��)