��Ŀ����
����Ŀ��
�����������ϳ��ֵĵ�һ�ֺϳ���ά�������ij���ʹ��֯Ʒ����ò��Ȼһ�£����ĺϳ��Ǻϳ���ά��ҵ���ش�ͻ�ƣ�ͬʱҲ�Ǹ߷��ӻ�ѧ��һ����Ҫ��̱�����ͼ����1,3������ϩΪԭ�����ϳ�����66��·�ߣ�

��֪��

��A����˳���칹
����������Ϣ�ش��������⣺
��1�����й����л��߷��ӻ������˵����ȷ����________��
A.�л��߷��ӻ��������ˮ��ΪС����
B.���ۺ���ά�صķ���ʽ��Ϊ(C6H10O5)n�����ǻ�Ϊͬ���칹��
C.�����ʵ����������������仯
D.�����ڸ������ױ��ԣ��������籣��ʱ�����
��2���л���A�Ľṹ��ʽΪ_______________��A��B�ķ�Ӧ����Ϊ___________��
��3��D�����������ŵ�����Ϊ_______________________��
��4��д��C+E��F�Ļ�ѧ����ʽ______________��
��5����E��������ͬ��ͬ���칹����______�֣�������E����д�����к˴Ź���������4�����շ��ҷ����֮��Ϊ2:4:1:3�Ľṹ��ʽ________________��
��6����֪��

��CH2=CH2Ϊԭ�ϣ��������Լ���ѡ�ϳ� 
��ϱ�����Ϣд���ϳ�·��_____________________________��
���𰸡� CD ![]() ȡ����Ӧ ̼̼˫�����Ȼ�
ȡ����Ӧ ̼̼˫�����Ȼ�  8
8 ![]()

����������������66�Ľṹ������֪��������1��3������ϩ��Cl2�ӳ�����A.![]() ��A��HCNȡ������B.
��A��HCNȡ������B.![]() ��B��H2�ӳ�����C.H2N(CH2)6NH2��B��H+/H2O������ˮ������D.
��B��H2�ӳ�����C.H2N(CH2)6NH2��B��H+/H2O������ˮ������D.![]() ��D��H2�ӳ�����E.HOOC(CH2)4COOH��E��C������������66��
��D��H2�ӳ�����E.HOOC(CH2)4COOH��E��C������������66��
��1��A�һ��ӳɾۺ��ﶼû��ˮ�⣬�������ϩ����1��3������ϩ���������ϩ�ȣ��������Ͼۺ������ˮ�⣬����۶Ա��������Ҷ��������ڣ����������������������ȣ�A����B�����ʽ(C6H10O5)n�е�nֵ��ȷ�������ۺ���ά�ز���ͬ���칹����B����C������ʵ������ǵ�������Һ�м�Ũ������Һ��ʹ������������û�����������������������仯��C��ȷ��D���������������ߵ����ʣ��������ڸ������ױ��ԣ����Ա���ʱ�������D��ȷ�����ϣ�ѡCD��
��2��������ķ����ɵã��л���A�Ľṹ��ʽΪ![]() ��A��B����ȡ����Ӧ��
��A��B����ȡ����Ӧ��
��3��DΪ![]() ������������Ϊ��̼̼˫�����Ȼ���
������������Ϊ��̼̼˫�����Ȼ���
��4��CΪH2N(CH2)6NH2��EΪHOOC(CH2)4COOH��C��E��������F����ѧ����ʽΪ�� ��
��
��5��EΪHOOC(CH2)4COOH����ͼ��ʾ�����λ�����Ȼ���������![]() ��
��![]() ��
��![]() ��
��![]() ��
��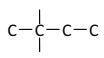 ��
��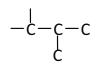 ��
��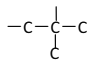 ��
��![]() ����E��������ͬ��ͬ���칹�壨������E����8�֣����к˴Ź���������4�����շ��ҷ����֮��Ϊ2:4:1:3�Ľṹ��ʽΪ��
����E��������ͬ��ͬ���칹�壨������E����8�֣����к˴Ź���������4�����շ��ҷ����֮��Ϊ2:4:1:3�Ľṹ��ʽΪ��![]() ��
��
 ���ϳ�
���ϳ� 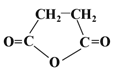 ����Ҫ
����Ҫ![]() ������֪
������֪![]() ��
��![]() ����CH2=CH2Ϊԭ�ϣ��ϳ�
����CH2=CH2Ϊԭ�ϣ��ϳ�  �ĺϳ�·��Ϊ��
�ĺϳ�·��Ϊ�� ��
��