��Ŀ����
A��B��C��D��EΪԪ�����ڱ��ж�����Ԫ���γɵ��������ӣ�A��B��C��D�������ӵĵ���������E�ĵ�������8��
��1��A��B��ϳɵĻ�������м������Ӽ����й��ۼ���A��C��ϳɵĻ�������������ˮ�������ڼ�ˮ��Һ����ɫ����Һ��CԪ�������ڱ��е�λ��Ϊ _ �������ˮ��Һ��Ӧ�����ӷ���ʽΪ__________________________________��
��2��B��E��ϳɻ������������������֮��Ϊ2��1��A��D��ϳɻ����ﶡ���������Ӧ������ɫ��ζ�����壬����ĵ���ʽΪ�� _ ��1molD���������� _ mol����
��3����һ��Һ̬���⻯�����죬�������E�ĵ�������ͬ���ǡ������ߺš��ɴ�����ʱʹ�õĸ���ȼ��֮һ���ṹ�������ָ÷��ӽṹ��ֻ�е������백���ƣ�����ĽṹʽΪ ___��
��1��A��B��ϳɵĻ�������м������Ӽ����й��ۼ���A��C��ϳɵĻ�������������ˮ�������ڼ�ˮ��Һ����ɫ����Һ��CԪ�������ڱ��е�λ��Ϊ _ �������ˮ��Һ��Ӧ�����ӷ���ʽΪ__________________________________��
��2��B��E��ϳɻ������������������֮��Ϊ2��1��A��D��ϳɻ����ﶡ���������Ӧ������ɫ��ζ�����壬����ĵ���ʽΪ�� _ ��1molD���������� _ mol����
��3����һ��Һ̬���⻯�����죬�������E�ĵ�������ͬ���ǡ������ߺš��ɴ�����ʱʹ�õĸ���ȼ��֮һ���ṹ�������ָ÷��ӽṹ��ֻ�е������백���ƣ�����ĽṹʽΪ ___��
��1����3���ڢ�A Al(OH)3+OH-=AlO2-+2H2O
��2�� 11mol
11mol
��3��
��2��
 11mol
11mol ��3��

�����������1����������Ϊ10�����ӵ������ӣ�N3-��O2-��F-��OH-��NH2-�� �����ӣ�Na+��Mg2+��Al3+��NH4+��H3O+����������Ϊ18�����ӵ������ӣ�S2-��O22-��Cl-��HS-�� �����ӣ�K+��Ca2+��N2H5+��N2H62+�����������⣬A��B��C��D����10e-���ӣ�EΪ18e-���ӣ�A��B�γɵĻ�������м������Ӽ����й��ۼ���10e-�������γɵĻ�����������Ӽ����й��ۼ�������Ϊ���λ��������A��C��ϳɵĻ�������������ˮ��10e-�����н�ϳ�������ˮ�Ļ�������Mg��OH��2��Al��OH��3���ٸ��������ڼ�ˮ��Һ����ɫ����Һ��ȷ����ΪAl��OH��3����Ϊǿ��NaOH����AΪOH-��BΪNa+��CΪAl3+����C�����ڱ��е�λ�õ������ڣ���A�塣Al��OH��3��NaOH��Ӧ����ʽΪAl��OH��3+NaOH�TNaAlO2+2H2O����д���ӷ���ʽΪAl��OH��3+OH-=AlO2-+2H2O����2���������⣬A��OH-��D��H3O+����ϳɻ����ﶡ����˶�ΪH2O��B��Na+��B��E�γɵĻ������������������֮��Ϊ2��1��E����ΪS2-��O22-������Ϊ�������Ӧ������ɫ��ζ���壬���Ա�ֻ��ΪNa2O2������ʽ
 1mol H3O+��11mol���ӡ�
1mol H3O+��11mol���ӡ���3���������⣬��������ΪҺ̬���⻯���ͬʱҲΪ18���ӣ�Nԭ�Ӻ�����7�����ӣ�Hԭ�Ӻ�����1�����ӣ���˻���������Ӧ��2��Nԭ�Ӻ�4��Hԭ�ӣ��ʻ�ѧʽΪN2H4���ṹ�����õ��÷�����ֻ���е��������Ե�ԭ�Ӻ͵�ԭ�ӡ���ԭ�Ӻ���ԭ��֮�䶼���γ�һ�Թ��õ��Ӷԣ��ʵ���ʽΪ
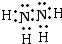 ���ṹʽ
���ṹʽ ��
��
��ϰ��ϵ�д�
 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д�
�����Ŀ
 Si(s)+2CO(g)
Si(s)+2CO(g) SiHCl3(l)��H2(g) + Q��Q��0��
SiHCl3(l)��H2(g) + Q��Q��0�� Si(��)��3HCl(g)
Si(��)��3HCl(g) ����Ҫ�ĺ˹�ҵԭ�ϣ����й���Dԭ�ӵ�˵���������
����Ҫ�ĺ˹�ҵԭ�ϣ����й���Dԭ�ӵ�˵��������� ԭ�Ӻ�����1������
ԭ�Ӻ�����1������ ������Ϊ2
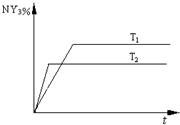
������Ϊ2 2NY3��g�����¶ȷֱ�ΪT1��T2ʱ��NY3�����������ʱ��仯����ͼ���÷�Ӧ�ġ�H 0�����������<����=������ͬ������T1��T2ʱ�÷�Ӧ�Ļ�ѧƽ�ⳣ���ֱ�ΪK1��K2����K1 K2��
2NY3��g�����¶ȷֱ�ΪT1��T2ʱ��NY3�����������ʱ��仯����ͼ���÷�Ӧ�ġ�H 0�����������<����=������ͬ������T1��T2ʱ�÷�Ӧ�Ļ�ѧƽ�ⳣ���ֱ�ΪK1��K2����K1 K2��