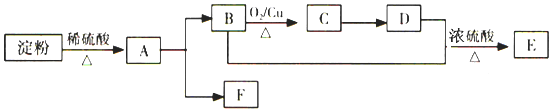
��Ŀ����
����Ŀ��X��Y��Z��M��R��W��Ϊ���ڱ���ǰ�����ڵ�Ԫ�أ�X�Ļ�̬ԭ����Χ�����Ų�ʽΪ3s2��Yԭ�ӵ�L���Ӳ��p�ܼ�����һ���չ����ZԪ�صĻ�̬ԭ���������3��δ�ɶԵ��ӣ��������2�����ӣ�M��ԭ�ӵ�2p�������1�����ӵ������������������ӵ����������෴��R�Ǻ�ˮ�г��⡢��Ԫ���⺬������Ԫ�أ�WΪ����Ԫ�أ����Ļ�̬ԭ����Χ�����Ų��ɶԵ�������δ�ɶԵ�������ͬ��Ϊ�������������������ش���������(��ػش����Ԫ�ط��ű�ʾ)��
��1��W�Ļ�̬ԭ�ӵ���Χ�����Ų�ͼΪ__�����̬ԭ����__��������ͬ�ĵ��ӡ�
��2��R���⻯��ķе������һ����ͬ��Ԫ���⻯��ķе�͵�ԭ����__��
��3��ZM3-�ռ乹��Ϊ___������Z���ӻ���ʽΪ__��
��4��W��YM���γ������W(YM)5����W(YM)5��W�Ļ��ϼ�Ϊ___����YM���ӻ�Ϊ�ȵ���������ӵĻ�ѧʽΪ___��
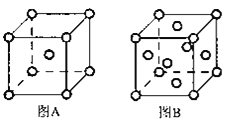
��5��W���ʵľ����ڲ�ͬ�¶���������ԭ�Ӷѻ���ʽ�������ֱ���ͼA��B��ʾ��ͼB��ԭ�Ӷѻ���ʽΪ___��A��B��Wԭ�ӵ���λ��֮��Ϊ___��A��B�������ⳤ�ֱ�Ϊacm��bcm����A��B���־�����ܶ�֮��Ϊ___��
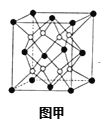
��6��X��W��ɵĺϽ���Ŀǰ�ѷ��ֵĴ����ܶ���ߵĴ������֮һ���侧���ṹ��ͼ��(�������W���������X).��úϽ�Ļ�ѧʽΪ___��
���𰸡�![]() 7 HF�����д������ ƽ�������� sp2 0 CN- ���������ܶѻ� 1��2 b3��2a3 FeMg2��Mg2Fe
7 HF�����д������ ƽ�������� sp2 0 CN- ���������ܶѻ� 1��2 b3��2a3 FeMg2��Mg2Fe
��������
X��Y��Z��M��R��W��Ϊ���ڱ���ǰ�����ڵ�Ԫ�أ�X�Ļ�̬ԭ����Χ�����Ų�ʽΪ![]() ����XΪ����������A��Ԫ�أ���XΪMgԪ�أ�Y��̬ԭ�ӵ�L���Ӳ��p�ܼ�����һ���չ����ԭ�Ӻ�������Ų�Ϊ
����XΪ����������A��Ԫ�أ���XΪMgԪ�أ�Y��̬ԭ�ӵ�L���Ӳ��p�ܼ�����һ���չ����ԭ�Ӻ�������Ų�Ϊ![]() ����YΪCԪ�أ�ZԪ�صĻ�̬ԭ���������3��δ�ɶԵ��ӣ��������2�����ӣ���Z���ڵڶ����ڣ������Ų�ʽΪ
����YΪCԪ�أ�ZԪ�صĻ�̬ԭ���������3��δ�ɶԵ��ӣ��������2�����ӣ���Z���ڵڶ����ڣ������Ų�ʽΪ![]() ����ZΪNԪ�أ�M�Ļ�̬ԭ�ӵ�2p�������1�����ӵ������������������ӵ����������෴��ԭ�Ӻ�������Ų�Ϊ
����ZΪNԪ�أ�M�Ļ�̬ԭ�ӵ�2p�������1�����ӵ������������������ӵ����������෴��ԭ�Ӻ�������Ų�Ϊ![]() ����MΪOԪ�أ�R�Ǻ�ˮ�г��⡢��Ԫ���⺬������Ԫ�أ���RΪClԪ�أ�WΪ����Ԫ�أ����Ļ�̬ԭ����Χ�����Ų��ɶԵ�������δ�ɶԵ�������ͬ��Ϊ����������������������Χ�����Ų�ʽΪ
����MΪOԪ�أ�R�Ǻ�ˮ�г��⡢��Ԫ���⺬������Ԫ�أ���RΪClԪ�أ�WΪ����Ԫ�أ����Ļ�̬ԭ����Χ�����Ų��ɶԵ�������δ�ɶԵ�������ͬ��Ϊ����������������������Χ�����Ų�ʽΪ![]() ����������Ų�Ϊ��
����������Ų�Ϊ��![]() ����WΪFeԪ�ء�
����WΪFeԪ�ء�
��1��WΪFeԪ�أ�FeΪ26��Ԫ�أ�������������Ϊ26����������Ų�Ϊ��![]() �����̬ԭ�ӵ���Χ�����Ų�ͼΪ��
�����̬ԭ�ӵ���Χ�����Ų�ͼΪ��![]() �����Ļ�̬ԭ����7���ܼ�����7��������ͬ�ĵ��ӣ� �ʴ�Ϊ��
�����Ļ�̬ԭ����7���ܼ�����7��������ͬ�ĵ��ӣ� �ʴ�Ϊ��![]() ��7��
��7��
��2��RΪClԪ�أ�Cl���⻯��Ϊ�Ȼ��⣬����һ����ͬ��Ԫ���⻯��ΪHF�����ڷ���������д�����������Է�����ķе�����Ȼ��⣬ �ʴ�Ϊ��HF�����д��������
��3��ZM3-Ϊ![]() ��
��![]() ��Nԭ�ӵļ۲���Ӷ���
��Nԭ�ӵļ۲���Ӷ���![]() ��û�йµ��Ӷԣ�����
��û�йµ��Ӷԣ�����![]() �Ŀռ乹��Ϊƽ�������Σ�Nԭ�Ӳ���
�Ŀռ乹��Ϊƽ�������Σ�Nԭ�Ӳ���![]() ���ʣ��ʴ�Ϊ��ƽ�������Σ�
���ʣ��ʴ�Ϊ��ƽ�������Σ�![]() ��
��
��4��W��YM���γ������![]() Ϊ
Ϊ![]() ��Fe��CO���γ������
��Fe��CO���γ������![]() ���ϼ۵Ĵ�����Ϊ��CO�Ļ��ϼ۵Ĵ�����Ϊ0����Fe�Ļ��ϼ�Ϊ0�����ݵȵ�����Ķ��壬CO�ĵȵ����������˫ԭ�ӷ��ӻ����ӣ��ҵ���������ȣ�������CO���ӻ�Ϊ�ȵ���������ӵĻ�ѧʽΪ
���ϼ۵Ĵ�����Ϊ��CO�Ļ��ϼ۵Ĵ�����Ϊ0����Fe�Ļ��ϼ�Ϊ0�����ݵȵ�����Ķ��壬CO�ĵȵ����������˫ԭ�ӷ��ӻ����ӣ��ҵ���������ȣ�������CO���ӻ�Ϊ�ȵ���������ӵĻ�ѧʽΪ![]() ���ʴ�Ϊ��0��
���ʴ�Ϊ��0��![]() ��
��
��5��A��ԭ��ռ�����ĺͶ��㣬Ϊ���������ṹ��ԭ����Ϊ![]() ��B��ռ�ݶ�������ģ�ԭ����Ϊ
��B��ռ�ݶ�������ģ�ԭ����Ϊ![]() ����������������������������������ʵ�ʺ��е�Feԭ�Ӹ���֮��1��2��A��B�������ⳤ�ֱ�Ϊacm��bcm������ֱ�Ϊ
����������������������������������ʵ�ʺ��е�Feԭ�Ӹ���֮��1��2��A��B�������ⳤ�ֱ�Ϊacm��bcm������ֱ�Ϊ![]() ��
��![]() ���������ܶȵ��ھ�������ԭ�ӵ�����������ı�Ϊ
���������ܶȵ��ھ�������ԭ�ӵ�����������ı�Ϊ![]() ��
��![]() ��
��![]() ���ʴ�Ϊ������������1��2��
���ʴ�Ϊ������������1��2��![]() ��
��![]() ��
��
��6��Feλ�����ĺͶ��㣬��ĿΪ��![]() ��Mgԭ��λ�����ģ���ĿΪ8����ѧʽΪ��
��Mgԭ��λ�����ģ���ĿΪ8����ѧʽΪ��![]() ��
��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��![]() ��
��
������� | �µ��Ӷ��� | �ӻ���ʽ | ���幹�� | ʾ�� |
AB2 | 0 | sp | ֱ���� | BeCl2 |
1 | sp2 | V�� | SO2 | |
2 | sp3 | V�� | H2O | |
AB3 | 0 | sp2 | ƽ�������� | BF3 |
1 | sp3 | ������ | NH3 | |
AB4 | 0 | sp3 | ���������� | CH4 |

 ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д�
ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д�����Ŀ�������������������������������Ҫ�����á��ش��������⣺
(1)��֪4NH3(g)+5O2(g)=4NO(g)+6H2O(g)��H1=��alkJ/mol��4NH3(g)+6NO(g)=5N2(g)+6H2O(g)��H2=��bkJ/mol��H2O(1)=H2O(g)��H3=+ckJ/mol��д����298Kʱ������ȼ������N2���Ȼ�ѧ����ʽ___________��
(2)�����еļ��쵰��(Mb)����O2�������MbO2��Mb(aq)+O2(g)![]() MbO2(aq)������k����k���ֱ��ʾ����Ӧ���淴Ӧ�����ʳ�������V��=k����c(Mb)��P(O2)��V��=k����c(MbO2)��37��ʱ��ü��쵰�Ľ�϶�(��)��P(O2)�Ĺ�ϵ���±�[��϶�(��)ָ����O2��ϵļ��쵰��ռ�ܼ��쵰�İٷֱ�]��
MbO2(aq)������k����k���ֱ��ʾ����Ӧ���淴Ӧ�����ʳ�������V��=k����c(Mb)��P(O2)��V��=k����c(MbO2)��37��ʱ��ü��쵰�Ľ�϶�(��)��P(O2)�Ĺ�ϵ���±�[��϶�(��)ָ����O2��ϵļ��쵰��ռ�ܼ��쵰�İٷֱ�]��
P(O2) | 0.50 | 1.00 | 2.00 | 3.00 | 4.00 | 5.00 | 6.00 |
����MbO2%�� | 50.0 | 67.0 | 80.0 | 85.0 | 88.0 | 90.3 | 91.0 |
�ټ���37�桢P(O2)Ϊ2.00kPaʱ��������Ӧ��ƽ�ⳣ��K=___________��
�ڵ���ƽ��ʱ���쵰����O2�Ľ�϶�(��)��O2��ѹǿ[P(O2)]֮��Ĺ�ϵʽ��=___________(�ú���k����k����ʽ�ӱ�ʾ)��
(3)���ɼ��쵰�ĸʰ���(NH2CH2COOH)��һ���������ʣ�����Һ��������������ʽ���ڣ���ת����ϵ���£�
![]()
![]()
![]()
![]()
![]()
�ڸʰ�����Һ�м�������������ӵİٷֺ�����![]() �Ĺ�ϵ��ͼ��ʾ��
�Ĺ�ϵ��ͼ��ʾ��

�ٴ��ʰ�����Һ��___________�ԣ�����Һ������ʱ�������ӵ�Ũ���ɴ�С��˳��Ϊ___________��
����![]() =8����Һ�м������HClʱ����Ӧ�����ӷ���ʽΪ___________��
=8����Һ�м������HClʱ����Ӧ�����ӷ���ʽΪ___________��
���õ�λ�ζ����ɲⶨij�ʰ�����Ʒ�Ĵ���.

��ȡ��Ʒ150mg����һ�������£���0.1000mol/L�ĸ�������Һ�ζ�(��ʰ���1�U1������Ӧ)����õ�ѹ�仯�����HClO4��Һ�������ϵ����ͼ�����հ���ʵ�飬����HClO4��Һ�����Ϊ0.25mL������Ʒ�Ĵ���Ϊ___________%(����������һλС��)

����Ŀ��(1)ijѧ���ñ�����ζ������NaOH��Һ������3��ʵ��ֱ��¼�й����������
�ζ����� | ����NaOH��Һ�����/mL | 0.1000mol/L��������/mL | ||
�ζ�ǰ�̶� | �ζ���̶� | ��Һ��� | ||
��һ�� | 25.00 | 0.00 | 26.11 | 26.11 |
�ڶ��� | 25.00 | 1.56 | 30.30 | 28.74 |
������ | 25.00 | 0.22 | 26.31 | 26.09 |
�����ݱ������ݣ���NaOH��Һ�����ʵ���Ũ��Ϊ______��
(2)ʵ�����ñ�������Һ�ⶨijNaOH��Һ��Ũ�ȣ��ü�����ָʾ�������в�������ʹ�ⶨ���ƫ�͵���______��
A.��ʽ�ζ���������ˮϴ�Ӻ�δ�ñ�Һ��ϴ
B.��ʼʵ��ʱ����ʽ�ζ��ܼ��첿�������ݣ��ڵζ�������������ʧ
C.�ζ������У���ƿ����Һ��ɫ�ɻ�ɫ��Ϊ��ɫ������������Ϊ��ɫ����ʱ��ֹͣ�ζ�����¼����
D.�ﵽ�ζ��յ�ʱ�����Ӷ�������¼
(3)ȷ��ȡ25.00mL���Ը��������ҺӦ��______��(����������)