��Ŀ����
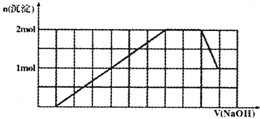
 ij��Һ�п��ܺ���H+��K+��NH4+��Mg2+��Fe3+��Al3+��SO42-��CO32-�����ӣ��������Һ�м���һ�����ʵ���Ũ�ȵ�NaOH��Һʱ���������ɳ������ʵ�����NaOH��Һ������仯��ͼ��ʾ���ɴ˿�֪
ij��Һ�п��ܺ���H+��K+��NH4+��Mg2+��Fe3+��Al3+��SO42-��CO32-�����ӣ��������Һ�м���һ�����ʵ���Ũ�ȵ�NaOH��Һʱ���������ɳ������ʵ�����NaOH��Һ������仯��ͼ��ʾ���ɴ˿�֪��1������Һ�п϶����е���������
H+��NH4+��Fe3+��Al3+
H+��NH4+��Fe3+��Al3+
������Щ�����ӵ����ʵ���֮��Ϊ1��2��1��1
1��2��1��1
���϶�������������������Mg2+
Mg2+
����2������һ�������ӿ��ܴ��ڣ�д�������������ӵ�ʵ�鷽��������
��ɫ��Ӧ������ɫ�겣���۲쵽�������ɫ
��ɫ��Ӧ������ɫ�겣���۲쵽�������ɫ
����������1������ͼ���һ�Σ������������ϳ��֣���֪һ���������ӣ�����кͷ�Ӧ��һ�з�Ӧ�����ȷ�Ӧ�����ɳ����ں����ܽ�һ���֣�һ���������ӣ����ݵ����Σ����������Ʒ�Ӧ�����Ӳ�����������֤����笠����ӣ�������������ɳ�������6������������ƣ��������������ܽ�����һ������������ƣ������γ���������������������������ƣ��������������������һ���DZ��������������ģ�����һ���������������ӣ�һ������̼������Ӻ�þ���ӣ���ԭ��Һ�к��е���������H+��NH4+��Fe3+��Al3+������ͼ������ϵ����Ϸ�Ӧ��Ҫ���������Ƶ�����������������ʵ���֮�ȣ�
��2�����������������Ӽ����ӿ��ܺ��У�������ɫ��Ӧ�����жϣ�
��2�����������������Ӽ����ӿ��ܺ��У�������ɫ��Ӧ�����жϣ�
����⣺��1������ͼ���һ�Σ������������ϳ��֣���֪һ���������ӣ�����кͷ�Ӧ��һ�з�Ӧ�����ȷ�Ӧ�����ɳ����ں����ܽ�һ���֣�һ���������ӣ����ݵ����Σ����������Ʒ�Ӧ�����Ӳ�����������֤����笠����ӣ�������������ɳ�������6������������ƣ��������������ܽ�����һ������������ƣ������γ���������������������������ƣ��������������������һ���DZ��������������ģ�����һ���������������ӣ�һ������̼������Ӻ�þ���ӣ���ԭ��Һ�к��е���������H+��NH4+��Fe3+��Al3+����Һ�е���غ��ж�������ֻ�ܺ���SO42-��������������ɳ�������6������������ƣ��������������ܽ�����1������������ƣ������γ�������������3������������ƣ�����3�������������һ���DZ��������������ģ�ԭ��Һ�к��е�Fe3+��Al3+�����ʵ���֮��Ϊ1��1���к�����������1����������ƣ���������笠����ӷ�Ӧ����2����������ƣ�����ͼ���� �Ķ�����ϵ����д�����ʵ���֮�ȣ�H+��NH4+��Fe3+��Al3+���ʵ���֮��Ϊ1��2��1��1��
�ʴ�Ϊ��H+��NH4+��Fe3+��Al3+��1��2��1��1��Mg2+��
��2��������Һ�е����ӵĴ��������֪���������Ӳ�һ�����ڣ�������ɫ��Ӧ�����жϣ���ɫ��Ӧ����ɫ�겣���۲쵽�������ɫ��֤����������ɫ֤�������ӣ�
�ʴ�Ϊ����ɫ��Ӧ������ɫ�겣���۲쵽�������ɫ��
�ʴ�Ϊ��H+��NH4+��Fe3+��Al3+��1��2��1��1��Mg2+��
��2��������Һ�е����ӵĴ��������֪���������Ӳ�һ�����ڣ�������ɫ��Ӧ�����жϣ���ɫ��Ӧ����ɫ�겣���۲쵽�������ɫ��֤����������ɫ֤�������ӣ�
�ʴ�Ϊ����ɫ��Ӧ������ɫ�겣���۲쵽�������ɫ��
������������һ���й����Ӽ�����ۺ�֪ʶ��Ŀ������ǶȺܹ㣬�ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ
 ij��Һ�п��ܺ���H+��Na+��NH4+��Mg2+��Fe3+��Al3+��SO42-��CO32-�����ӣ��������Һ�м���һ�����ʵ���Ũ�ȵ�NaOH��Һʱ���������ɳ������ʵ�����NaOH��Һ������仯��ͼ����ͼ��ʾ������˵����ȷ���ǣ�������
ij��Һ�п��ܺ���H+��Na+��NH4+��Mg2+��Fe3+��Al3+��SO42-��CO32-�����ӣ��������Һ�м���һ�����ʵ���Ũ�ȵ�NaOH��Һʱ���������ɳ������ʵ�����NaOH��Һ������仯��ͼ����ͼ��ʾ������˵����ȷ���ǣ�������| A��ԭ��Һ�к��е���������H+��NH4+��Mg2+��Al3+ | B��ԭ��Һ��һ������SO42-��Na+ | C��ԭ��Һ��SO42-�����ʵ�������Ϊ3.5mol | D����Ӧ����γɵ���Һ�к��е�����ΪNa2SO4 |
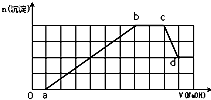 ij��Һ�п��ܺ���H+��NH4+��Mg2+��Fe3+��Al3+��SO42-��HCO3-�����ӣ��������Һ�м���һ�����ʵ���Ũ�ȵ�NaOH��Һʱ���������ɳ��������ʵ��������NaOH��Һ������仯��ͼ��ʾ������˵����ȷ���ǣ�������
ij��Һ�п��ܺ���H+��NH4+��Mg2+��Fe3+��Al3+��SO42-��HCO3-�����ӣ��������Һ�м���һ�����ʵ���Ũ�ȵ�NaOH��Һʱ���������ɳ��������ʵ��������NaOH��Һ������仯��ͼ��ʾ������˵����ȷ���ǣ�������| A��d����Һ�к��е�����ֻ��Na2SO4 | B��ԭ��Һ�к��е�Fe3+��Al3+�����ʵ���֮��Ϊ1��1 | C��ab�η��������ӷ�ӦΪ��Al3++3OH-=Al��OH��3����Mg2++2OH-=Mg��OH��2�� | D��ԭ��Һ�к��е������ӱض���H+��NH4+��Al3+�������ܿ϶�Mg2+��Fe3+�е���һ�� |
 ��2012?���Ķ�ģ��ij��Һ�п��ܺ���H+��Na+��NH4+��Mg2+��Fe3+��Al3+��SO42-��CO32-�����ӣ��������Һ�м���һ�����ʵ���Ũ�ȵ�NaOH��Һʱ���������ɳ��������ʵ�����NaOH��Һ������仯��ͼ����ͼ��ʾ������˵����ȷ���ǣ�������
��2012?���Ķ�ģ��ij��Һ�п��ܺ���H+��Na+��NH4+��Mg2+��Fe3+��Al3+��SO42-��CO32-�����ӣ��������Һ�м���һ�����ʵ���Ũ�ȵ�NaOH��Һʱ���������ɳ��������ʵ�����NaOH��Һ������仯��ͼ����ͼ��ʾ������˵����ȷ���ǣ�������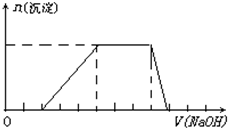 ij��Һ�п��ܺ���H+��Na+��NH4+��Mg2+��Fe3+��Al3+��SO42-�����ӣ��������Һ�м���ijŨ�ȵ�NaOH��Һʱ���������ɳ��������ʵ�����NaOH��Һ����ı仯��ͼ��ʾ���ɴ˿�֪������Һ��һ�����е���������
ij��Һ�п��ܺ���H+��Na+��NH4+��Mg2+��Fe3+��Al3+��SO42-�����ӣ��������Һ�м���ijŨ�ȵ�NaOH��Һʱ���������ɳ��������ʵ�����NaOH��Һ����ı仯��ͼ��ʾ���ɴ˿�֪������Һ��һ�����е���������