��Ŀ����
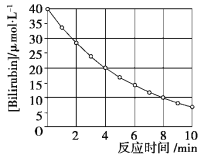
����Ŀ���������Ƴ���Ư����ɱ���������������������Ʊ��治���������տ�����CO2�����ʡ�ij����С��Ϊ�˴��Բⶨ�������ƵĴ��ȣ����dz�ȡa g��Ʒ�����������ͼװ�����ⶨ�������Ƶ�����������
�����������Ӻ��Ժ�����еĵ�һ��������_____��
��Bװ�ó����������Ƿ���Ҫ����______����������������������
��D��NaOH��Һ������_________��
��ʵ�����ʱ����ȡʵ����������������ʱ������������_____��
a��ֱ�Ӷ�ȡ���������������ȴ������
b�������ƶ���Ͳ��ʹ��E��F��Һ��߶���ͬ
c�������밼Һ�����͵���ƽ��ȡ��Ͳ��ˮ�����
��������Ͳ��ˮ�����������ɱ�״�������������ΪV mL������Ʒ�й������Ƶ���������Ϊ____��
��ʵ����ɺ�E��F֮�䵼���ڲ���ˮ�������ʹ�������________������ƫ������ƫС��������Ӱ��������
���𰸡����װ�õ������� �� ����δ��Ӧ��CO2 a ![]() % ƫС
% ƫС
��������
�ⶨ�������ƵĴ��ȣ�Aװ�ã���ȡ������̼��A�з�����Ӧ��̼��ƺ����ᷴӦ���ɶ�����̼��ˮ���Ȼ��ƣ�Bװ�ã�ϴ�������ն�����̼�л��е�HCl����ֹHCl��������Ʒ�Ӧ��Cװ�ã�������̼��ˮ�����������Ʒ�Ӧ����Ӧ�Ļ�ѧ����ʽΪ��2CO2+2Na2O2=2Na2CO3+O2��2Na2O2+2H2O=4NaOH+O2����Dװ�ã����ն���Ķ�����̼��E��Fװ�ã���ˮ�������塣
װ��ͼ��AΪ���ɶ�����̼��װ�ã�̼��ƺ����ᷴӦ���ɶ�����̼��ˮ���Ȼ��ƣ���Ӧ�����ӷ���ʽΪ��CaCO3+2H+=Ca2++H2O+CO2����
BΪϴ��װ�ã����ն�����̼�л��е�HCl����ֹHCl��������Ʒ�Ӧ��CΪ������̼��������Ʒ�Ӧ��װ�ã�DΪ���ն���Ķ�����̼��װ�ã���ֹ����Ķ�����̼��������װ�ã����²�õ����������ƫ��E��F�Dz������������������װ�ã�
��ʵ��̽���ⶨ�����Dzⶨ������̼�������Ʒ�Ӧ���ɵ�������װ���б�������������ã����������Ӻ��Ժ�����еĵ�һ�������Ǽ��װ�õ������ԣ�
��Bװ�ó��������岻��Ҫ���������̼��ˮ�����������Ʒ�Ӧ����̼���ƺ������ķ�Ӧ����Ӧ�Ļ�ѧ����ʽΪ��2CO2+2Na2O2=2Na2CO3+O2��2Na2O2+2H2O=4NaOH+O2�����������Ʒ�Ӧ�������������ʵ���������ϵ��ͬ�Բⶨ������������������Ӱ�죬���Բ���Ҫ�����ȥˮ������
��DΪ���ն���Ķ�����̼��װ�ã���ֹ����Ķ�����̼��������װ�ã����²�õ����������ƫ��
��a��ֱ�Ӷ�ȡ�������������ȴ�����£���ʹ��Һ��������������������a����ȷ��
b��������Ͳ����Һ��߶�ʹ֮��ͬ��ʹװ����ѹǿ�����ѹǿ��ͬ�������ȡ�����������b��ȷ��
c�������밼Һ�����͵���ƽ��ȡ��Ͳ��ˮ���������ȷ�Ķ�ȡ��������c��ȷ��
�ʴ�Ϊ��a��
�ݲⶨ����Ͳ��ˮ�����������ɱ�״�������������ΪVmL�����ʵ���=![]() =
=![]() mol������Ʒ�й������Ƶ���������Ϊ=
mol������Ʒ�й������Ƶ���������Ϊ= ��100%=
��100%=![]() %��
%��
��ʵ����ɺ�E��F֮�䵼���ڲ���ˮ�������ʹ�ⶨ���������С�����²ⶨ�����������������������ƫС��

����Ŀ������(HCOOH)�ǻ�ԭ���ᣬ�ֳ������ᣬ��������ҽҩ��Ⱦ�ϡ�Ƥ��ȹ�ҵ������ij��ѧ��ȤС���ڷ����������ɺͽṹ�Լ����ijЩ���ʽ�����̽������ش��������⡣
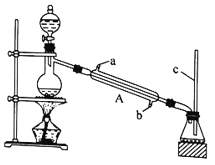
I.�������봼����������Ӧ
����ȤС��������ͼ��ʾװ�ý��м���(HCOOH)��״�(CH3OH)��������Ӧʵ��:
�й����ʵ���������:
�е�/�� | �ܶ�(g��cm-3) | ˮ���ܽ��� | |
�״� | 64.5 | 0.79 | ���� |
���� | 100.7 | 1.22 | ���� |
������� | 31.5 | 0.98 | ���� |
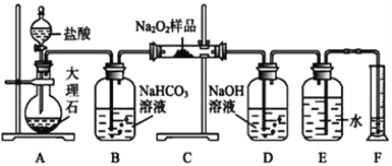
(1)װ���У�����A��������_________________����������c��������__________________________��
(2)����ͼ״�����������Ӧ�Ļ�ѧ����ʽΪ____________________________________________��
(3)Ҫ����ƿ�����õĻ��������ȡ����������ɲ��õķ���Ϊ__________________________________��
II.��������ˮ��ȡһ����̼

(1)������ͼװ���Ʊ����ռ�CO����������ȷ������˳��Ϊa��__________(�������������������Сд��ĸ��ʾ)��
(2)װ��B��������__________________________________��
(3)һ�������£�CO����NaOH���巢����Ӧ:CO+NaOH ![]() HCOONa��
HCOONa��
��Ϊ��֤����CO��NaOH���巢���˷�Ӧ����������ж���ʵ�鷽��:ȡ�����������Һ��___________��
���ⶨ�����м�����(HCOONa)�Ĵ�����ȷ��ȡ�������8,0g���Ƴ�100mL��Һ����ȡ20.00mL����Һ����ƿ�����ټ���___________��ָʾ������1.5mol/L���������Һ�ζ�ʣ���NaOH��ƽ�еζ����Σ�ƽ��������������Ϊ5.05mL��������м����Ƶ���������Ϊ_______(��������ȷ��0.1%)��