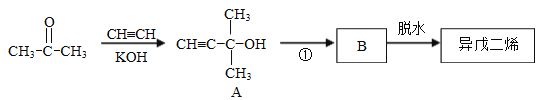��Ŀ����
����Ŀ��ʵ����ģ�ҵ��ȡNa2SO3����Ĺ������£�

��֪���� ��ӦI��������ƿ�з�����װ����ͼ����ʾ���̶�������������ʡ�ԣ���
�� �������ʵ��ܽ��������ͼ����ʾ������Na2SO3������Һ����50��ʱ����Na2SO3��7H2O��
��l����ӦI��Ŀ������ȡ(NH4)2SO3��Һ��

�� ��ӦI�����ӷ���ʽΪ____________��
�� ������ֽ������SO2����ȴ����ͨ�백ˮ�У�Ŀ����____________��
�� ���й���ͼ��װ�û������������ȷ����______������ĸ����
A�����������ܵ���ȴˮ��a��ͨ��
B�����������ܾ��з�����������
C. ���Ƽ�����������¶ȣ����Կ�������SO2���������
��2��Ϊ��ȡ�����Na2SO3���壬Ҫ����ӦII���¶ȿ�����80�����ң���_______�������I�����ƣ���
��3������Һ������ȡNH4Cl���塣
����֤��Һ�к���NH4+��ʵ�������________��
������Һ�ɻ��NH4Cl�ֲ�Ʒ��������Na2SO3�����벹��������NH4Cl�ֲ�Ʒ��ȡ������NH4Cl�����ʵ�鷽����________���õ�������NH4Cl���塣��ʵ������ʹ�õ��Լ���SO2���Ҵ�����������������ʹ�õ������У���ո����䣩
���𰸡� 2NH3��H2O+SO2=2NH4++SO32-+H2O ����SO2���ܽ�ȣ����SO2�������� BC ���ȹ��� ȡ������Һ���Թ��У������еμ�NaOH��Һ�������Թܣ���ʪ��ĺ�ɫʯ����ֽ���ڹܿڣ���ֽ���� ��NH4Cl�ֲ�Ʒ��������ˮ����������Һ��ͨ��������SO2���壻��������Һ����Ũ������ȴ�ᾧ�����ˣ������������Ҵ�ϴ��2--3�κ�����ո������и���
����������l����ӦI��Ŀ������ȡ(NH4)2SO3��Һ���� ��ӦI�����ӷ���ʽΪ2NH3��H2O+SO2=2NH4++SO32-+H2O;�� ������ܽ�����¶ȵ����߶����ͣ�������ֽ������SO2����ȴ����ͨ�백ˮ�У�Ŀ���� ����SO2���ܽ�ȣ����SO2�������ʣ��� A�����������ܵ���ȴˮ��b��ͨ�룬��A����B�����������ܾ��з����������ã���B��ȷ��C�������¶ȿ��Լӿ췴Ӧ���ʣ����Ƽ�����������¶ȣ����Կ�������SO2��������ʣ���C��ȷ����ѡBC����2��Na2SO3������Һ����50��ʱ����Na2SO3��7H2O����ֹ����Na2SO3��7H2O��Ҫ���ȹ��ˣ���3������NH4�� ��ȡ������Һ���Թ��У������еμ�NaOH��Һ�������Թܣ���ʪ��ĺ�ɫʯ����ֽ���ڹܿڣ���ֽ���� ������NH4Cl�ֲ�Ʒ��ȡ������NH4Cl�����ʵ�鷽������NH4Cl�ֲ�Ʒ��������ˮ����������Һ��ͨ��������SO2���壻��������Һ����Ũ������ȴ�ᾧ�����ˣ������������Ҵ�ϴ��2--3�κ�����ո������и���õ�������NH4Cl���塣

 ��У����ϵ�д�
��У����ϵ�д�