��Ŀ����
2����CH4����ԭNO��������������������ۣ������������β����Ⱦ���⣬��Ӧ���£�CH4��g��+4NO2��g��=4NO��g��+CO2��g��+2H2O��g������H=-574kJ•mol-1
CH4��g��+4NO��g��=2N2��g��+CO2��g��+2H2O��g������H=-akJ•mol-1
������ת������״����44.8L NO2����ԭ��N2�����������зų�������Ϊ867kJ����a��ֵΪ��������
| A�� | 1160 | B�� | 2308 | C�� | 1441 | D�� | 2320 |
���� �Ƚ���������ʽ��ӵ� NO2ת��Ϊ�����ķ���ʽ�����ݱ�״����44.8L NO2����ԭ��N2�����������зų�������Ϊ867kJ����a��ֵ��
��� �⣺����������ʽCH4��g��+4NO2��g��=4NO��g��+CO2��g��+2H2O��g����H1=-574kJ•mol-1��CH4��g��+4NO��g��=2N2��g��+CO2��g��+2H2O��g����H2=-akJ•mol-1��ӵã�2CH4��g��+4NO2��g��=2N2��g��+2CO2��g��+4H2O��g����H=-��574+a��kJ•mol-1
�ֱ�״����44.8L NO2����ԭ��N2����2molNO2����ԭ��N2�ų�������Ϊ867kJ������867��2=574+a�����a=1160����ѡ��A��
���� ���⿼���˷�Ӧ�ȵļ����֪ʶ�㣬�������ʵ����������Ĺ�ϵʽ����ų����������ɣ��ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
19��Ϊ�˲ⶨijδ֪NaOH��Һ��Ũ�ȣ���Ҫ0.200mol•L-1��������Һ500mL��ijѧ����ʵ�����г��õ�36.5%���ܶ�Ϊ1.20g•cm-3����Ũ������������ϡ���ᣬ����������²������ʵ�飮
��1�����������ͬѧ������¸����е����ݣ�����������Ƶ�ʵ�鲽����ȷ����
A����b�������±�ѡ������ѡȡ�ʵ���������������룩������ƣ�ȡ36.5%���ܶ�Ϊ1.19g•cm-3����Ũ����8.3ml����д�����������������
a.50mL��Ͳ�� b.10mL��Ͳ�� c��������ƽ��
B����ȡ�õ�Ũ�������ձ��м���������ˮϡ�ͣ�
C�����������������ˮ��Һ�������������1��2cm����
D������Һ�ָ������£�
E������Һ�ò���������ת��500ml����ƿ����д�����������У�������������ˮϴ���ձ��Ͳ�����2��3��һ��ת�����У�
F���ý�ͷ�ιܼ�����ˮֱ����ҺҺ��ǡ����������У�����ƿ��ҡ�ȼ��ɣ�
���ϸ�ʵ�鲽�����ȷ����˳����ABDECF��
��2��ȷ��ȡ20.00mL����NaOH��Һ��һ�ྻ��ƿ�У�Ȼ���������������Һ���еζ����Է�̪Ϊָʾ�������ζ�������±���
�����������ݼ����δ֪NaOH��Һ�����ʵ���Ũ��Ϊ0.22mol•L-1����ȷ��0.01����
��3�����в�����������ȷ����BC����д��ţ���
A���ζ������У��۾�ע�ӵζ�������Һ��ʹ������Һ��İ���ˮƽ��
B���ζ�����������ҡ����ƿʹ������Һ����մ��ƿ�ڱڣ�Ϊʹ�ⶨ�����ȷ��������������ˮ���������ƿ�ڵ���Һ�У�
C������ƿ�͵ζ�����ʹ��ǰϴ����Ҫ����Ƿ�©Һ��
D������ƿ����ȡNaOH��Һǰ��������NaOH��Һ��ϴ��ƿ2��3�Σ�
��4�����в���������ᵼ�²ⶨ���ƫ�ߵ���ACE����д��ţ���
A���ζ������в�С�Ľ�һ����Һ������ƿ�⣻
B���ζ�����ʱ�������ڵζ��ܼ��촦��С���ݣ�
C���ζ����������ӵζ���Һ�������
D����Һ��ɫ����������ҡ����ƿ����Һ��ɫ������
E����ʽ�ζ���������ˮϴ����δ�ñ�������ϴ��ֱ�Ӽ����Һ���еζ���
��1�����������ͬѧ������¸����е����ݣ�����������Ƶ�ʵ�鲽����ȷ����
A����b�������±�ѡ������ѡȡ�ʵ���������������룩������ƣ�ȡ36.5%���ܶ�Ϊ1.19g•cm-3����Ũ����8.3ml����д�����������������
a.50mL��Ͳ�� b.10mL��Ͳ�� c��������ƽ��
B����ȡ�õ�Ũ�������ձ��м���������ˮϡ�ͣ�
C�����������������ˮ��Һ�������������1��2cm����
D������Һ�ָ������£�
E������Һ�ò���������ת��500ml����ƿ����д�����������У�������������ˮϴ���ձ��Ͳ�����2��3��һ��ת�����У�
F���ý�ͷ�ιܼ�����ˮֱ����ҺҺ��ǡ����������У�����ƿ��ҡ�ȼ��ɣ�
���ϸ�ʵ�鲽�����ȷ����˳����ABDECF��
| ʵ����� | �ζ���Һ����ʼ���� | �ζ���Һ���յ���� |
| 1 | 1.32mL | 23.36mL |
| 2 | 2.26mL | 24.22mL |
�����������ݼ����δ֪NaOH��Һ�����ʵ���Ũ��Ϊ0.22mol•L-1����ȷ��0.01����
��3�����в�����������ȷ����BC����д��ţ���
A���ζ������У��۾�ע�ӵζ�������Һ��ʹ������Һ��İ���ˮƽ��
B���ζ�����������ҡ����ƿʹ������Һ����մ��ƿ�ڱڣ�Ϊʹ�ⶨ�����ȷ��������������ˮ���������ƿ�ڵ���Һ�У�
C������ƿ�͵ζ�����ʹ��ǰϴ����Ҫ����Ƿ�©Һ��
D������ƿ����ȡNaOH��Һǰ��������NaOH��Һ��ϴ��ƿ2��3�Σ�
��4�����в���������ᵼ�²ⶨ���ƫ�ߵ���ACE����д��ţ���
A���ζ������в�С�Ľ�һ����Һ������ƿ�⣻
B���ζ�����ʱ�������ڵζ��ܼ��촦��С���ݣ�
C���ζ����������ӵζ���Һ�������
D����Һ��ɫ����������ҡ����ƿ����Һ��ɫ������
E����ʽ�ζ���������ˮϴ����δ�ñ�������ϴ��ֱ�Ӽ����Һ���еζ���
13������˵����ȷ���ǣ�������
| A�� | �ڳ��³�ѹ�£�11.2L N2���еķ�����Ϊ0.5NA | |
| B�� | 32g O2��ԭ����ĿΪ2NA | |
| C�� | ��״���£�18g H2O��ռ�����Ϊ22.4L | |
| D�� | ��ͬ��ͬѹ�£���ͬ����κ����嵥��������ԭ����Ŀ��ͬ |
10�����л������У��������ԣ����ܷ����ӳɷ�Ӧ��������Ӧ��������Ӧ����ȥ��Ӧ���ǣ�������
| A�� | 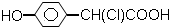 | B�� | CH3CH ��OH��-CH=CH-COOH | ||
| C�� | CH3-CH=CH-COOH | D�� | CH3CH��OH��CH2CHO |
7������������˵������ȷ���ǣ�������
| A�� | ��ʪ��ĺ�ɫʯ����ֽ���鰱�� | |
| B�� | ������ͭ��Ũ���ᷴӦ��һ���������� | |
| C�� | �������Ż�ʱ�����ö�����̼�������� | |
| D�� | Na2CO3�����ȶ��Դ���NaHCO3 |
14�����й��ڷ����廯����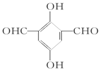 ˵����ȷ���ǣ�������
˵����ȷ���ǣ�������
 ˵����ȷ���ǣ�������
˵����ȷ���ǣ�������| A�� | 1 molA����4 mol Ag��NH3��2OH��Һ����������Ӧ | |
| B�� | �˴Ź�������ͼ��ʾ���������շ� | |
| C�� | ������FeCl3��Һ������ɫ��Ӧ | |
| D�� | 1 mol A����2 mol NaHCO3��ȫ��Ӧ�ų�CO2 |
12�����л���ԭ�ϵ�ѡ�������ɫ��ѧ˼����ǣ�������
| A�� | ʹ��������ԭ�� | B�� | ʹ����ɫΪ��ɫ�Ļ�����Ʒ | ||
| C�� | ��ʹ���κλ�ѧ���� | D�� | ʹ�ò�����������Դ |
 ��
��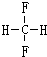
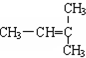 ��CH2=CH-CH2
��CH2=CH-CH2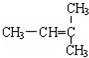 ��
��
