��Ŀ����
����Ŀ��H2S��SO2��Ի��������彡�����������Σ������ҵ�ϲ�ȡ���ַ���������Щ�к�������ŷţ��ش����и������е����⡣
��H2S�ij�ȥ
����1��������H2S��ԭ��Ϊ��
H2S+Fe2(SO4)3![]() S��+2FeSO4+H2SO4
S��+2FeSO4+H2SO4
4FeSO4+O2+2H2SO4![]() 2Fe2(SO4)3+2H2O
2Fe2(SO4)3+2H2O
��1����˾�����ʱ��FeSO4����������������ʱ��5��105�����þ���������______________��
��2����ͼ3��ͼ4�ж�ʹ����˾����������Ϊ______________������Ӧ�¶ȹ��ߣ���Ӧ�����½�����ԭ����______________��

����2����һ�������£���H2O2����H2S
��3�����Ųμӷ�Ӧ��n(H2O2)/n(H2S)�仯���������ﲻͬ����n(H2O2)/n(H2S)=4ʱ����������ķ���ʽΪ__________��
��SO2�ij�ȥ
����1��˫�������NaOH����SO2������CaOʹNaOH����
NaOH��Һ![]() Na2SO3
Na2SO3
��4��д�����̢ٵ����ӷ���ʽ��____________________________��CaO��ˮ�д�������ת����
CaO(s)+H2O (l) ![]() Ca(OH)2(s)
Ca(OH)2(s)![]() Ca2+(aq)+2OH(aq)
Ca2+(aq)+2OH(aq)
��ƽ���ƶ��ĽǶȣ��������̢�NaOH������ԭ��____________________________________��
����2���ð�ˮ��ȥSO2
��5����֪25�棬NH3��H2O��Kb=1.8��105��H2SO3��Ka1=1.3��102��Ka2=6.2��108������ˮ��Ũ��Ϊ2.0 mol��L-1����Һ�е�c(OH)=_________________mol��L1����SO2ͨ��ð�ˮ�У���c(OH)����1.0��107 mol��L1ʱ����Һ�е�c(![]() )/c(
)/c(![]() )=___________________��
)=___________________��
���𰸡� ���ͷ�Ӧ��ܣ����������� 30 �桢pH=2.0 �����ʱ��ԣ�����˾�ʧȥ���ԣ� H2SO4 2OH-+ SO2![]() SO32-+H2O SO32-��Ca2+����CaSO3������ƽ���������ƶ�����NaOH���� 6.0��10-3 0.62
SO32-+H2O SO32-��Ca2+����CaSO3������ƽ���������ƶ�����NaOH���� 6.0��10-3 0.62
����������1��������˾�����ʱ�ķ�Ӧ��������ʱ��5��105����֪����Ӧ����������������˾����������������������˷�Ӧ�Ļ�ܡ�
��2������ͼ3��֪���¶�30������ʱ���������������ͼ4��֪��pH=2.0ʱ���������������ʹ����˾����������Ϊ30�桢pH=2.0������Ӧ�¶ȹ��ߣ���˾������ʱ���ʧȥ������ɷ�Ӧ�����½���
��3�����ݻ��ϼ���������n(H2O2)/n(H2S)=4ʱ��4mol H2O2ת��8mol���ӣ���1mol H2SҲת��8mol���ӣ����ϼ۴�-2�����ߵ�+6�ۣ�������������ΪH2SO4��
��4�����̢���NaOH��SO2�ķ�Ӧ����Ӧ�����ӷ���ʽΪ2OH-+ SO2![]() SO32-+H2O������CaO��ˮ�е�ת����CaO(s)+ H2O (l)
SO32-+H2O������CaO��ˮ�е�ת����CaO(s)+ H2O (l) ![]() Ca(OH)2(s)
Ca(OH)2(s)![]() Ca2+(aq)+2OH(aq)�����̢���Na2SO3����CaO��SO32-��Ca2+����CaSO3������ƽ���������ƶ�����NaOH���ɡ�
Ca2+(aq)+2OH(aq)�����̢���Na2SO3����CaO��SO32-��Ca2+����CaSO3������ƽ���������ƶ�����NaOH���ɡ�
��5������NH3��H2O��Kb=1.8��105��֪��![]() =1.8��105������ˮ��Ũ��Ϊ2.0 mol��L-1ʱ����Һ�е�c(OH)= c(NH4+)=
=1.8��105������ˮ��Ũ��Ϊ2.0 mol��L-1ʱ����Һ�е�c(OH)= c(NH4+)=![]() =6.0��10-3 mol��L-1��
=6.0��10-3 mol��L-1��
����H2SO3��Ka2=6.2��108��֪��![]() =6.2��108������c(OH)����1.0��107 mol��L1����Һ�е�c(SO32-)/c(HSO3-)=0.62��
=6.2��108������c(OH)����1.0��107 mol��L1����Һ�е�c(SO32-)/c(HSO3-)=0.62��

����Ŀ��TiCl4�����Ѿ�����Ҫ�ɷ�ΪTiO2���Ʊ��ѣ�Ti������Ҫ�м����Ʊ���TiCl4������ʾ��ͼ���£�
![]()
���ϣ�TiCl4�����������Ȼ��������
������ | SiCl4 | TiCl4 | AlCl3 | FeCl3 | MgCl2 |
�е�/�� | 58 | 136 | 181�������� | 316 | 1412 |
�۵�/�� | 69 | 25 | 193 | 304 | 714 |
��TiCl4�е��ܽ��� | ���� | ���� | �� | ���� | |
��1���Ȼ����̣�TiO2��Cl2����ֱ�ӷ�Ӧ����̼����CO��CO2��ʹ��Ӧ���Խ��С�
��֪��TiO2(s)+2 Cl2(g)= TiCl4(g)+ O2(g) ��H1=+175.4 kJ��mol-1
2C(s)+O2(g)=2CO(g) ��H2=-220.9 kJ��mol-1
�� ����¯�м�̼�Ȼ�����TiCl4(g)��CO(g)���Ȼ�ѧ����ʽ��____________________��
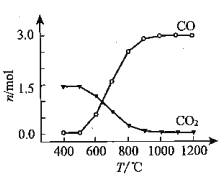
�� �Ȼ�������CO��CO2�����ת����������ͼ�жϣ�CO2����CO��Ӧ�Ħ�H_____0���������������=�������ж����ݣ�_______________��
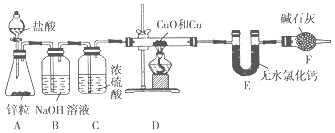
�� �Ȼ���Ӧ��β���봦�����ŷţ�β���е�HCl��Cl2�����տɵô����ᡢFeCl3��Һ����β��������Һ������__________________________��
�� �Ȼ�������ȴ�����£������˵õ���TiCl4���Һ���������к���_____________��
��2�����ƹ��̣���TiCl4����������ô�TiCl4��ʾ��ͼ���£�
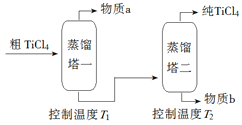
����a��______________��T2Ӧ������_________��
����Ŀ���������ʵķ�����ȷ����(����)
ѡ�� | �� | �� | �� | ���������� | ���������� |
A | Na2CO3 | H2SO4 | NaOH | SO2 | CO2 |
B | NaOH | HCl | NaCl | Na2O | NO |
C | KOH | HNO3 | CaCO3 | CaO | Mn2O7 |
D | NaOH | HCl | CaF2 | Na2O2 | SO2 |
A. A B. B C. C D. D