��Ŀ����
3���衢������Ļ���������������������������е���ϵ����ش��������⣺��1�����ά����Ϊ��Ϣ���ٹ�·�ĹǼܣ�����Ҫ�ɷ��Ƕ��������SiO2
��2�����Ļ������кܶ࣬���м�������ˮ�ҳ�����������������ǰ�����NH3
��3������ʱ��Ũ�������̼������Ӧ��C+2H2SO4��Ũ��=CO2��+2SO2��+2H2O���ڸ÷�Ӧ�У�ŨH2SO4���ֳ����������ˮ��������ˮ�������������ԣ�
���� ��1�����ά����Ҫ�ɷ��Ƕ������裻
��2������Һ���������մ������ȣ��ܹ�������
��3�����ݷ���ʽ��Ũ��������Ԫ�ػ��ϼ۱仯���
��� �⣺��1�����ά����Ҫ�ɷ��Ƕ������裬
�ʴ�Ϊ�����������SiO2��
��2�������е�ߣ�Һ���������մ������ȣ��������������
�ʴ�Ϊ��������NH3��
��3���ڷ�ӦC+2H2SO4��Ũ��=CO2��+2SO2��+2H2O��Ũ�����е���Ԫ�ػ��ϼ�ȫ�����ͣ�����Ũ������������ԣ�����������
�ʴ�Ϊ��������
���� ���⿼�������ʵ����ʺ���;����Ϥ�������衢���������ʣ���ϤŨ��������ʼ��ɽ����Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ����С��ʿ���������ϵ�д�
����С��ʿ���������ϵ�д�
�����Ŀ
14�������£�����������ȷ���ǣ�������
| A�� | pH=5.6����CH3COOH��CH3COONa��ɵĻ����Һ�У�c��Na+����c��CH3C00-�� | |
| B�� | ��PH=a�Ĵ���ϡ��ΪpH=a+1�Ĺ����У�C��OH-���������� | |
| C�� | �����pH=a��������pH=b�İ�ˮ��Һǡ���к�ʱ��a+b=14 | |
| D�� | Ũ�Ⱦ�Ϊ0.1 mol•L-1��CH3COOH��Һ�Ͱ�ˮ�������Ϻ�c��CH3COO-��+c��H+��=c��NH4+��+c��OH-�� |
11�������йع������Ƶ������У�����ȷ���ǣ�������
| A�� | ��������Ϊ����ɫ���� | |
| B�� | ����������ˮ��Ӧ�Ƿ��ȷ�Ӧ | |
| C�� | �������ƿ����ں����������Ϊ��������Դ | |
| D�� | ���������������̼��Ӧ���û���Ӧ |
18����֪��ϩ�ܹ�ʹ��ˮ��ɫ�������ķ�Ӧ��CH2=CH2+Br2��CH2Br-CH2Br���÷�Ӧ���ڣ�������
| A�� | �ӳɷ�Ӧ | B�� | ȡ����Ӧ | C�� | ������Ӧ | D�� | ˮ�ⷴӦ |
8��һֻ�ɰ���Сè�������µ�վ��һ��߷��Ӻϳɲ��ϰ��ϣ������һ����գ�˵���ø߷��Ӳ��ϰ�һ�����е������ǣ�������
| A�� | ������ | B�� | ��Ե�� | C�� | ������ | D�� | �۵�� |
18����ͼ��ʾ��������G����ƫת��ͬʱA����֣�B����ϸ��CΪ�������Һ����A��B��CΪ��������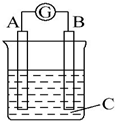

| A�� | A��Zn��B��Cu��C��ϡ���� | B�� | A��Cu��B��Zn��C��ϡ���� | ||
| C�� | A��Fe��B��Ag��C��ϡAgNO3��Һ | D�� | A��Ag��B��Fe��C��ϡAgNO3��Һ |
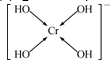 ��������λ�����ü��ű�ʾ��
��������λ�����ü��ű�ʾ��
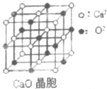 ���������з�Ӧ�ϳ������谷��CaO+3C$\frac{\underline{\;����\;}}{\;}$CaC2+CO����CaC2+N2$\frac{\underline{\;����\;}}{\;}$CaCN2+C��
���������з�Ӧ�ϳ������谷��CaO+3C$\frac{\underline{\;����\;}}{\;}$CaC2+CO����CaC2+N2$\frac{\underline{\;����\;}}{\;}$CaCN2+C��