��Ŀ����
ij��ѧѧϰС��ͬѧ��ʵ������ȡ�����Ͱ����Ļ�ԭ�ԵȽ���������̽����������벢��ɶ��й��� ��Ľ��
[���ϻ�Ϥ]
�ٰ�����CuO��Ӧ�Ļ�ѧ����ʽ��3CuO+2NH3 3Cu+N2+3H2O
3Cu+N2+3H2O
��Cu2O��ĩ�ʺ�ɫ��Cu2O�Ǽ����������������Һ��Cu+���ȶ�����ת��ΪCu ��Cu2+ ��Cu+ Cu+Cu2+ ��
Cu+Cu2+ ��
���ڿ����и�������ʱ��Cu2O�ȶ������ֽ⣬��CuO���ֽ�����Cu2O��O2��
[ʵ�����]
[���ϻ�Ϥ]
�ٰ�����CuO��Ӧ�Ļ�ѧ����ʽ��3CuO+2NH3
 3Cu+N2+3H2O
3Cu+N2+3H2O ��Cu2O��ĩ�ʺ�ɫ��Cu2O�Ǽ����������������Һ��Cu+���ȶ�����ת��ΪCu ��Cu2+ ��Cu+
 Cu+Cu2+ ��
Cu+Cu2+ �� ���ڿ����и�������ʱ��Cu2O�ȶ������ֽ⣬��CuO���ֽ�����Cu2O��O2��
[ʵ�����]
[ʵ��̽��]
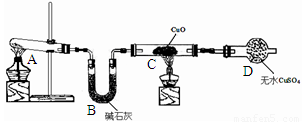
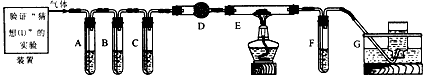
��1��Aװ���ǰ����ķ���װ�ã���ʵ��ǰ���Թ���Ӧ�����ҩƷ��____________��
��2����ͬѧ��Ϊ��ʵ�������һ����ȱ�ݣ�Aװ�ò����İ����к���ˮ������Ӱ����ʵ����ۣ�Ӧ��A��B֮������һ��װ��___________����ҩƷ���ƣ��ĸ���װ�á�
��3�����øĽ����װ�ý���ʵ�飬�ɹ۲쵽Bװ���е�����Ϊ��ɫ�����ɺ�ɫ��Cװ���е�����Ϊ____________ ��
[��������]
��4����ͬѧ��ΪNH3��CuO��Ӧ���ɵĺ�ɫ�����п��ܺ���Cu2O(������ͭ)������Ũ���ᡢϡ���ᡢϡ���ᡢ����������Һ��pH��ֽ���������ʵ�鷽��֤����ɫ�������Ƿ���Cu2O��Ҫѡ�õ��Լ���____________��
��5����ͬѧ��Ϊ��ͨ���������պ�ɫ���壬��������Ӧǰ����������ı仯��ȷ���Ƿ���Cu2O�����ȳƸ�������������Ϊa g����ȡ��ɫ�������������гƵ�������Ϊb g���ڿ����и��������������㶨�����Ƶ�������Ϊc g����ȷ�Ϻ�ɫ������ֻ����Cu���������պ�õ���������____________����ȷ�Ϻ�ɫ�����к���Cu2O����a��b��cӦ������ѧ��ϵΪc< ____________ ��
[��չ����]
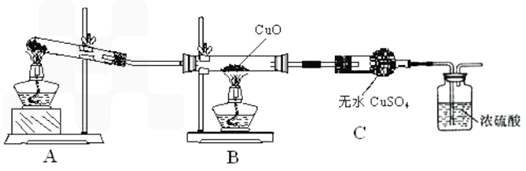
��6����ͬѧ��Ϊ��������ʵ����ƸĽ����װ�û��ɲⶨCu�����ԭ������������ͨ���ⶨ��Ӧ��CuO��������������H2O����������ɵġ���ʵ������ȫ��Ӧ��CuO�����ɵ�H2O�������ֱ�Ϊm(CuO)��m(H2O)����ݴ˼���Cu�����ԭ�������Ĵ���ʽΪ_________��
��1��Aװ���ǰ����ķ���װ�ã���ʵ��ǰ���Թ���Ӧ�����ҩƷ��____________��
��2����ͬѧ��Ϊ��ʵ�������һ����ȱ�ݣ�Aװ�ò����İ����к���ˮ������Ӱ����ʵ����ۣ�Ӧ��A��B֮������һ��װ��___________����ҩƷ���ƣ��ĸ���װ�á�
��3�����øĽ����װ�ý���ʵ�飬�ɹ۲쵽Bװ���е�����Ϊ��ɫ�����ɺ�ɫ��Cװ���е�����Ϊ____________ ��
[��������]
��4����ͬѧ��ΪNH3��CuO��Ӧ���ɵĺ�ɫ�����п��ܺ���Cu2O(������ͭ)������Ũ���ᡢϡ���ᡢϡ���ᡢ����������Һ��pH��ֽ���������ʵ�鷽��֤����ɫ�������Ƿ���Cu2O��Ҫѡ�õ��Լ���____________��
��5����ͬѧ��Ϊ��ͨ���������պ�ɫ���壬��������Ӧǰ����������ı仯��ȷ���Ƿ���Cu2O�����ȳƸ�������������Ϊa g����ȡ��ɫ�������������гƵ�������Ϊb g���ڿ����и��������������㶨�����Ƶ�������Ϊc g����ȷ�Ϻ�ɫ������ֻ����Cu���������պ�õ���������____________����ȷ�Ϻ�ɫ�����к���Cu2O����a��b��cӦ������ѧ��ϵΪc< ____________ ��
[��չ����]
��6����ͬѧ��Ϊ��������ʵ����ƸĽ����װ�û��ɲⶨCu�����ԭ������������ͨ���ⶨ��Ӧ��CuO��������������H2O����������ɵġ���ʵ������ȫ��Ӧ��CuO�����ɵ�H2O�������ֱ�Ϊm(CuO)��m(H2O)����ݴ˼���Cu�����ԭ�������Ĵ���ʽΪ_________��
��1��NH4Cl �� Ca(OH)2
��2����ʯ��
��3����ˮCuSO4�����ɫ
��4��ϡH2SO4
��5��Cu2O ��
��6��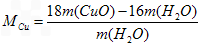
��2����ʯ��
��3����ˮCuSO4�����ɫ
��4��ϡH2SO4
��5��Cu2O ��

��6��
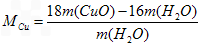

��ϰ��ϵ�д�
�����Ŀ