��Ŀ����
X��Y��Z��W����Ԫ�ص�ԭ���������������Ҿ�Ϊ������Ԫ�أ�Xԭ�������������Ǵ�����������2����Y�����ֳ�����ͬ�������壬����һ���Ǻܺõ���ɫ������������Z��Wԭ������������֮����Xԭ��������������2����������Ԫ�ص�ԭ�ӵ��Ӳ���֮��Ϊ10������գ�
��1��X��Y���һ���г�ζ�����壬�����������ʵ���֮��Ϊ1��2��Ϻ�ǡ����ȫȼ�գ������ȶ����������ͬ��ͬѹ�²��ȼ��ǰ���������������䣬�÷�Ӧ�Ļ�ѧ����ʽΪ______����̬��XY2����______���壮
��2����W�ĵ������ж����壬������ǿ����Һ��Ӧ�����ӷ���ʽΪ______����Z��Y�ɹ��ɾ���Ư�����õĻ���������ʽΪ______��
��3����W�ĵ���Ϊ����ɫ���壬��W��X���γɻ�����XW2��
��XW2Ϊ______������ԡ��Ǽ��ԡ������ӣ�
��Z�ĵ�����XY2������ȼ�յ�����Ϊ______
��4����W��һ�ֵ��ʷ���Ϊ������ṹ������������ȼ�յ�����Ļ���ȣ�ʵ�����������õ���Ӧ������______�У�ͨ��ʵ������ȡZ������������ˮ��������ӳ�ʽΪ______��
��1��X��Y���һ���г�ζ�����壬�����������ʵ���֮��Ϊ1��2��Ϻ�ǡ����ȫȼ�գ������ȶ����������ͬ��ͬѹ�²��ȼ��ǰ���������������䣬�÷�Ӧ�Ļ�ѧ����ʽΪ______����̬��XY2����______���壮
��2����W�ĵ������ж����壬������ǿ����Һ��Ӧ�����ӷ���ʽΪ______����Z��Y�ɹ��ɾ���Ư�����õĻ���������ʽΪ______��
��3����W�ĵ���Ϊ����ɫ���壬��W��X���γɻ�����XW2��
��XW2Ϊ______������ԡ��Ǽ��ԡ������ӣ�
��Z�ĵ�����XY2������ȼ�յ�����Ϊ______
��4����W��һ�ֵ��ʷ���Ϊ������ṹ������������ȼ�յ�����Ļ���ȣ�ʵ�����������õ���Ӧ������______�У�ͨ��ʵ������ȡZ������������ˮ��������ӳ�ʽΪ______��
X��Y��Z��W����Ԫ�ص�ԭ���������������Ҿ�Ϊ������Ԫ�أ�Xԭ�������������Ǵ�����������2������XΪ̼Ԫ�أ�Y�����ֳ�����ͬ�������壬����һ���Ǻܺõ���ɫ��������������YΪ��Ԫ�أ�Z��Wԭ������������֮����Xԭ��������������2����������Ԫ�ص�ԭ�ӵ��Ӳ���֮��Ϊ10����ZΪ�������ڵڢ�A��Ԫ�أ���ZΪ��Ԫ�أ�WΪ�������ڵ�
����A��Ԫ�أ���WΪ��Ԫ�ط������⣻��ZΪ�������ڵڢ�A��Ԫ�أ���ZΪþԪ�أ�WΪ�������ڵڢ�A��Ԫ�أ���WΪ��Ԫ��Ҳ�������⣻��ZΪ�������ڵڢ�A��Ԫ�أ���ZΪ��Ԫ�أ�WΪ�������ڵڢ�A��Ԫ�أ���WΪ��Ԫ��Ҳ�������⣻
��1����̼�����γɵĻ�����ΪCxOy����CxOy+2O2�T3CO2���������غ㶨�ɿ�֪x=3��y=2������ѧ��ӦΪC3O2+2O2�T3CO2��������̼�Ĺ������Ƿ��ӣ����̬�Ķ�����̼���ڷ��Ӿ��壬�ʴ�Ϊ��C3O2+2O2�T3CO2�����ӣ�
��2��W�ĵ������ж����壬��õ���Ϊ������������Ӧ�����ӷ���ʽΪCl2+2OH-=Cl-+ClO-+H2O��Z��Y�ɹ��ɾ���Ư�����õĻ�����Ϊ���������������ӻ�����������ӹ��ɵģ������ʽΪ
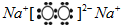
���ʴ�Ϊ��Cl2+2OH-=Cl-+ClO-+H2O��
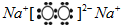
��
��3����W�ĵ���Ϊ����ɫ���壬��W�ĵ���ΪS����CO2��CS2���ƣ�����ֱ���ͷ��ӣ��ṹ�Գƣ�������ɵ������غϣ������ڷǼ��Է��ӣ��ʴ�Ϊ���Ǽ��ԣ�
��WΪ��Ԫ��ʱZΪþԪ�أ�þ�������̼��Ӧ��������þ��̼����ɹ۲쵽þ���ڶ�����̼�о���ȼ�գ����ɰ�ɫ��ĩ����ƿ���ڱ��к�ɫ���帽�ţ��ʴ�Ϊ��þ���ڶ�����̼�о���ȼ�գ����ɰ�ɫ��ĩ����ƿ���ڱ��к�ɫ���帽�ţ�
��4��W��һ�ֵ��ʷ���Ϊ������ṹ������������ȼ�յ�����Ļ���ȣ���WΪ���ף��ɱ�����ʢ����ˮ�Ĺ��ƿ�У�WΪ�ף���ZΪ����ʵ������ȡ��������һ�����ú��������ӵ���Һ�백ˮ��Ӧ�������ӷ�ӦΪAl3++3NH3?H2O=Al��OH��3��+3NH4+���ʴ�Ϊ��ʢ����ˮ�Ĺ��ƿ��Al3++3NH3?H2O=Al��OH��3��+3NH4+��
����A��Ԫ�أ���WΪ��Ԫ�ط������⣻��ZΪ�������ڵڢ�A��Ԫ�أ���ZΪþԪ�أ�WΪ�������ڵڢ�A��Ԫ�أ���WΪ��Ԫ��Ҳ�������⣻��ZΪ�������ڵڢ�A��Ԫ�أ���ZΪ��Ԫ�أ�WΪ�������ڵڢ�A��Ԫ�أ���WΪ��Ԫ��Ҳ�������⣻
��1����̼�����γɵĻ�����ΪCxOy����CxOy+2O2�T3CO2���������غ㶨�ɿ�֪x=3��y=2������ѧ��ӦΪC3O2+2O2�T3CO2��������̼�Ĺ������Ƿ��ӣ����̬�Ķ�����̼���ڷ��Ӿ��壬�ʴ�Ϊ��C3O2+2O2�T3CO2�����ӣ�
��2��W�ĵ������ж����壬��õ���Ϊ������������Ӧ�����ӷ���ʽΪCl2+2OH-=Cl-+ClO-+H2O��Z��Y�ɹ��ɾ���Ư�����õĻ�����Ϊ���������������ӻ�����������ӹ��ɵģ������ʽΪ
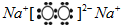
���ʴ�Ϊ��Cl2+2OH-=Cl-+ClO-+H2O��
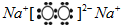
��
��3����W�ĵ���Ϊ����ɫ���壬��W�ĵ���ΪS����CO2��CS2���ƣ�����ֱ���ͷ��ӣ��ṹ�Գƣ�������ɵ������غϣ������ڷǼ��Է��ӣ��ʴ�Ϊ���Ǽ��ԣ�
��WΪ��Ԫ��ʱZΪþԪ�أ�þ�������̼��Ӧ��������þ��̼����ɹ۲쵽þ���ڶ�����̼�о���ȼ�գ����ɰ�ɫ��ĩ����ƿ���ڱ��к�ɫ���帽�ţ��ʴ�Ϊ��þ���ڶ�����̼�о���ȼ�գ����ɰ�ɫ��ĩ����ƿ���ڱ��к�ɫ���帽�ţ�
��4��W��һ�ֵ��ʷ���Ϊ������ṹ������������ȼ�յ�����Ļ���ȣ���WΪ���ף��ɱ�����ʢ����ˮ�Ĺ��ƿ�У�WΪ�ף���ZΪ����ʵ������ȡ��������һ�����ú��������ӵ���Һ�백ˮ��Ӧ�������ӷ�ӦΪAl3++3NH3?H2O=Al��OH��3��+3NH4+���ʴ�Ϊ��ʢ����ˮ�Ĺ��ƿ��Al3++3NH3?H2O=Al��OH��3��+3NH4+��

��ϰ��ϵ�д�
�����Ŀ



