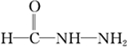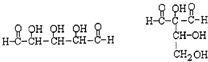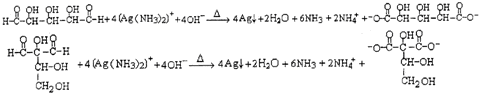��Ŀ����
��1����״���£����ԼΪ11.2L��NH3��Լ����
��2��ͬ��ͬѹ�£�ͬ�����İ������������壨H2S���������Ϊ
��3����ij�¶�ʱ��һ������Ԫ��A���⻯��AH3��һ������ܱ������п���ȫ�ֽ��������̬���ʣ���ʱ�ܱ���������������ܵ����ʵ���������75%����A���ʵ�һ����������
3.01��1024
3.01��1024
�����ӣ���2��ͬ��ͬѹ�£�ͬ�����İ������������壨H2S���������Ϊ
2��1
2��1
��ͬ��ͬѹ�£���������ԭ������ȣ����ǵ������Ϊ2��3
2��3
����3����ij�¶�ʱ��һ������Ԫ��A���⻯��AH3��һ������ܱ������п���ȫ�ֽ��������̬���ʣ���ʱ�ܱ���������������ܵ����ʵ���������75%����A���ʵ�һ����������
4
4
��Aԭ�ӣ�AH3�ֽⷴӦ�Ļ�ѧ����ʽΪ4AH3
A4+6H2
| ||
4AH3
A4+6H2
��
| ||
��������1������n=
=
��Ϸ��ӵĹ��ɼ��㣻
��2������n=
=
=
��Ϸ��ӵĹ��ɼ��㣻
��3����ֽ������Am������2mAH3
2Am+3mH2�����ݷ�Ӧǰ�����ʵ����Ĺ�ϵ���㣮
| V |
| Vm |
| N |
| NA |
��2������n=
| m |
| M |
| V |
| Vm |
| N |
| NA |
��3����ֽ������Am������2mAH3
| ||
����⣺��1��n��NH3��=
=0.5mol�����еĵ�����Ϊ��N=0.5mol��6.02��1023/mol��10=3.01��1024��
�ʴ�Ϊ��3.01��1024��
��2������n=
=
���жϣ�ͬ��ͬѹ�£�ͬ�����İ������������壨H2S���������Ϊ��Ħ�������ɷ��ȣ�
Ϊ34g/mol��17g/mol=2��1��ͬ��ͬѹ�£���������ԭ������ȣ��趼Ϊ6mol������Ϊ2mol������Ϊ3mol������n=
�����ǵ�����ȵ������ʵ���֮��Ϊ2��3��
�ʴ�Ϊ��2��1��2��3��
��3����ֽ������Am������2mAH3
2Am+3mH2���ܱ���������������ܵ����ʵ���������75%��
����
=1+0.75��
m=4��
�ɵ÷�Ӧ�ķ���ʽΪ4AH3
A4+6H2��
�ʴ�Ϊ��4��4AH3
A4+6H2��
| 11.2L |
| 22.4L/mol |
�ʴ�Ϊ��3.01��1024��
��2������n=
| m |
| M |
| V |
| Vm |
Ϊ34g/mol��17g/mol=2��1��ͬ��ͬѹ�£���������ԭ������ȣ��趼Ϊ6mol������Ϊ2mol������Ϊ3mol������n=
| V |
| Vm |
�ʴ�Ϊ��2��1��2��3��
��3����ֽ������Am������2mAH3
| ||
����
| 2+3m |
| 2m |
m=4��
�ɵ÷�Ӧ�ķ���ʽΪ4AH3
| ||
�ʴ�Ϊ��4��4AH3
| ||
���������⿼�����ʵ�������ؼ��㣬��Ŀ�Ѷ��еȣ�ע�������ؼ��㹫ʽ�����ã�Ϊ������Ĺؼ���

��ϰ��ϵ�д�
 ���ƿ�����ϵ�д�
���ƿ�����ϵ�д� ���¿쳵����������ϵ�д�
���¿쳵����������ϵ�д�
�����Ŀ
 ����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ��
����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ��