��Ŀ����
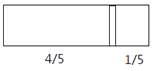
����Ŀ��һ���ܱ��������м���һ�����ɻ����ĸ��壨��Ȳ��ƣ��������ֳ������֣�����߳���8molN2���ұ߳���CO��CO2�Ļ�����干64gʱ�����崦����ͼλ�ã������¶Ȳ��䣩������˵����ȷ����
A.�ұ�CO��CO2������֮��Ϊ1:3
B.�ұ�CO������Ϊ14g
C.�ұ������ܶ�����ͬ�����������ܶȵ�2��
D.���ı��ұ�CO��CO2�ij�������ʹ���崦�ھ����Ҷ�1/3 �����������¶Ȳ��䣬��ǰ�����������ڵ�ѹǿ֮��Ϊ5:6
���𰸡�D
��������
��֪���ܱ��������м��������ɻ��������������������¶ȡ�ѹǿ��ͬ������ͬ�¡�ͬѹ�£���������֮�ȵ������ʵ���֮�ȣ��ݴ˼����Ҳ�CO��CO2�������ʵ������ٽ��CO��CO2������������CO��CO2�����ʵ������ݴ˽��
��֪���ܱ��������м��������ɻ��������������������¶ȡ�ѹǿ��ͬ������ͬ�¡�ͬѹ�£���������֮�ȵ������ʵ���֮�ȣ������������֮��Ϊ4:1����֪���������������ʵ���֮��Ϊ4:1�������Ҳ�CO��CO2�������ʵ���=![]() ����CO��CO2������Ϊ64g����CO�����ʵ���Ϊxmol���������̼���ʵ���Ϊ(2x)mol��28xg+44(2x)g=64g�����x=1.5mol����CO�����ʵ���Ϊ1.5mol��������̼���ʵ���Ϊ0.5mol��
����CO��CO2������Ϊ64g����CO�����ʵ���Ϊxmol���������̼���ʵ���Ϊ(2x)mol��28xg+44(2x)g=64g�����x=1.5mol����CO�����ʵ���Ϊ1.5mol��������̼���ʵ���Ϊ0.5mol��
A. ������������ʵ���֮�ȵ����������֮�ȣ���֪�ұ�CO��CO2������֮��Ϊ1.5mol:0.5mol=3:1����A����
B. m(CO)=n(CO)M(CO)=1.5mol��28g/mol=42g����B����
C. ��ͬ�����£������ܶ�֮�ȵ�����Ħ������֮�ȣ��ұ������ƽ��Ħ������![]() ��������Ħ��������ȣ���C����
��������Ħ��������ȣ���C����
D. ���ı��ұ�CO��CO2�ij�������ʹ���崦�ھ����Ҷ�![]() �����������������֮��Ϊ2:1������CO��CO2���ʵ���Ϊ4mol������ͬ�¡�ͬ�ݻ��£������ѹǿ֮�ȵ��������ʵ���֮�ȣ�������ѹǿ֮��Ϊ(8+2)mol:(8+4)mol=5:6����D��ȷ��
�����������������֮��Ϊ2:1������CO��CO2���ʵ���Ϊ4mol������ͬ�¡�ͬ�ݻ��£������ѹǿ֮�ȵ��������ʵ���֮�ȣ�������ѹǿ֮��Ϊ(8+2)mol:(8+4)mol=5:6����D��ȷ��
��ѡD��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ����3�������Ϊ2.0 L�ĺ����ܱ������У���ӦCO2(g)��C(s)![]() 2CO(g)��H>0���ֱ���һ���¶��´ﵽ��ѧƽ��״̬������˵����ȷ����
2CO(g)��H>0���ֱ���һ���¶��´ﵽ��ѧƽ��״̬������˵����ȷ����
���� | �¶�/K | ��ʼʱ���ʵ���/mol | ƽ��ʱ���ʵ���/mol | ||
n(CO2) | n(C) | n(CO) | n(CO) | ||
I | 977 | 0.28 | 0.56 | 0 | 0.4 |
II | 977 | 0.56 | 0.56 | 0 | x |
III | 1250 | 0 | 0 | 0.56 | y |
A.977K���÷�Ӧ�Ļ�ѧƽ�ⳣ��ֵΪ2
B.�ﵽƽ��ʱ��������I������C������ƽ�������ƶ�
C.�ﵽƽ��ʱ����������CO2��ת���ʱ��������еĴ�
D.�ﵽƽ��ʱ���������е�CO��ת���ʴ���28.6%
����Ŀ������ʵ������Լ�ʵ��������ȫһ�µ��ǣ� ��
A | ������ͭ��Һ�м���һС������� | �к�ɫ�������� |
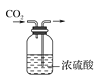
B | ��̼������Һ��ͨ����� | �а�ɫ�������� |
C | �����Ƶ���ˮ�ε���ɫʯ����ֽ�� | ��ֽ��� |
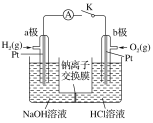
D | ����ɰֽ��ĥ�����������ھƾ��ƻ����ϼ��� | ���ۻ����������� |
A.AB.BC.CD.D