��Ŀ����
����Ҫ���������й����ӷ�Ӧ����Ŀ
��һ���Ӣ�Ba��OH��2����H2SO4����Zn����CuSO4
��CaCO3����HNO3����NaOH��ѡ�����ʵ����ʣ�ʵ�����и���Ҫ��ķ�Ӧ��
����ѡ�����ʵ����ʲ�д����ѧ��Ӧ�����ӷ���ʽ��
��1��ʵ������ȡCO2�ķ�Ӧ��
��2��ʵ������ȡH2�ķ�Ӧ��
����ѡ�����ʵ����ʲ�д�����������ӷ���ʽ���Ӧ�Ļ�ѧ����ʽ��
��3��Cu2++2OH-�TCu��OH��2��
��4��H++OH-�TH2O
��������A��B��C������ɫ��Һ��ֻ֪����HCl��H2SO4��Ba��NO3��2�������ʵ���Һ�еĸ�һ�֣���һ��˳�����Na2CO3��Һ�У���ǡ����ȫ��Ӧ������������
�ټ���Aʱ�����ɰ�ɫ������������г�������Bʱ�������ܽ⣬��������ų�������������ɵ���Һ�м���Cʱ�����а�ɫ�������ɣ�
�ж�A��B��C��Һ�����ʷֱ�Ϊ��A
��һ���Ӣ�Ba��OH��2����H2SO4����Zn����CuSO4
��CaCO3����HNO3����NaOH��ѡ�����ʵ����ʣ�ʵ�����и���Ҫ��ķ�Ӧ��
����ѡ�����ʵ����ʲ�д����ѧ��Ӧ�����ӷ���ʽ��
��1��ʵ������ȡCO2�ķ�Ӧ��
�ݢ�
�ݢ�
��CaCO3+2H+�TCa2++CO2��+H2O
CaCO3+2H+�TCa2++CO2��+H2O
����2��ʵ������ȡH2�ķ�Ӧ��
�ڢ�
�ڢ�
��Zn+2H+�TH2��+Zn2+
Zn+2H+�TH2��+Zn2+
������ѡ�����ʵ����ʲ�д�����������ӷ���ʽ���Ӧ�Ļ�ѧ����ʽ��
��3��Cu2++2OH-�TCu��OH��2��
�ܢ�
�ܢ�
��CuSO4+2NaOH�TCu��OH��2��+Na2SO4
CuSO4+2NaOH�TCu��OH��2��+Na2SO4
����4��H++OH-�TH2O
�ޢ�
�ޢ�
��HNO3+NaOH�TNaNO3+H2O
HNO3+NaOH�TNaNO3+H2O
����������A��B��C������ɫ��Һ��ֻ֪����HCl��H2SO4��Ba��NO3��2�������ʵ���Һ�еĸ�һ�֣���һ��˳�����Na2CO3��Һ�У���ǡ����ȫ��Ӧ������������
�ټ���Aʱ�����ɰ�ɫ������������г�������Bʱ�������ܽ⣬��������ų�������������ɵ���Һ�м���Cʱ�����а�ɫ�������ɣ�
�ж�A��B��C��Һ�����ʷֱ�Ϊ��A
Ba��NO3��2
Ba��NO3��2
��B��HCl
HCl
��C��H2SO4
H2SO4
����������һ��������1��ʵ�������������̼�����ȡ������̼��
��2��ʵ��������Zn��ϡ���ᷴӦ��ȡ������
������3��������ͭ����ǿ�Ӧ����������ͭ�Ϳ������ο���Cu2++2OH-�TCu��OH��2����ʾ��
��4��ǿ����ǿ�ᷴӦ���ɿ������κ�ˮ�����ӷ�ӦΪH++OH-�TH2O��
�������ټ���Aʱ�����ɰ�ɫ��������AΪBa��NO3��2������г�������Bʱ�������ܽ⣬��������ų�������������ɵ���Һ�м���Cʱ�����а�ɫ�������ɣ���BΪHCl��CΪH2SO4���Դ������
��2��ʵ��������Zn��ϡ���ᷴӦ��ȡ������
������3��������ͭ����ǿ�Ӧ����������ͭ�Ϳ������ο���Cu2++2OH-�TCu��OH��2����ʾ��
��4��ǿ����ǿ�ᷴӦ���ɿ������κ�ˮ�����ӷ�ӦΪH++OH-�TH2O��
�������ټ���Aʱ�����ɰ�ɫ��������AΪBa��NO3��2������г�������Bʱ�������ܽ⣬��������ų�������������ɵ���Һ�м���Cʱ�����а�ɫ�������ɣ���BΪHCl��CΪH2SO4���Դ������
����⣺��һ��������1��ʵ�������������̼�����ȡ������̼��ѡ���Լ�Ϊ�ݢޣ����ӷ�ӦΪCaCO3+2H+�TCa2++CO2��+H2O��
�ʴ�Ϊ���ݢޣ�CaCO3+2H+�TCa2++CO2��+H2O��
��2��ʵ��������Zn��ϡ���ᷴӦ��ȡ������ѡ���Լ�Ϊ�ڢۣ����ӷ�ӦΪZn+2H+�TH2��+Zn2+��
�ʴ�Ϊ���ڢۣ�Zn+2H+�TH2��+Zn2+��
������3��������ͭ����ǿ�Ӧ����������ͭ�Ϳ������ο���Cu2++2OH-�TCu��OH��2����ʾ����ѡ��ܢߣ���ѧ��ӦΪCuSO4+2NaOH�TCu��OH��2��+Na2SO4��
�ʴ�Ϊ���ܢߣ�CuSO4+2NaOH�TCu��OH��2��+Na2SO4��
��4��ǿ����ǿ�ᷴӦ���ɿ������κ�ˮ�����ӷ�ӦΪH++OH-�TH2O���Լ�Ϊ�ޢ�ڢ�٢ޣ���ѧ��ӦΪHNO3+NaOH�TNaNO3+H2O��H2SO4+2NaOH�T2H2O+Na2SO4��Ba��OH��2+2HNO3�T2H2O+Ba��NO3��2��
�ʴ�Ϊ���ޢߣ�HNO3+NaOH�TNaNO3+H2O��
���������ټ���Aʱ�����ɰ�ɫ����Ϊ̼�ᱵ����AΪBa��NO3��2������г�������Bʱ�������ܽ⣬��������ų�������Ϊ������̼������������ɵ���Һ�м���Cʱ�����а�ɫ�������ɣ��ð�ɫ����Ϊ���ᱵ����BΪHCl��CΪH2SO4���ʴ�Ϊ��Ba��NO3��2��HCl��H2SO4��
�ʴ�Ϊ���ݢޣ�CaCO3+2H+�TCa2++CO2��+H2O��
��2��ʵ��������Zn��ϡ���ᷴӦ��ȡ������ѡ���Լ�Ϊ�ڢۣ����ӷ�ӦΪZn+2H+�TH2��+Zn2+��
�ʴ�Ϊ���ڢۣ�Zn+2H+�TH2��+Zn2+��
������3��������ͭ����ǿ�Ӧ����������ͭ�Ϳ������ο���Cu2++2OH-�TCu��OH��2����ʾ����ѡ��ܢߣ���ѧ��ӦΪCuSO4+2NaOH�TCu��OH��2��+Na2SO4��
�ʴ�Ϊ���ܢߣ�CuSO4+2NaOH�TCu��OH��2��+Na2SO4��
��4��ǿ����ǿ�ᷴӦ���ɿ������κ�ˮ�����ӷ�ӦΪH++OH-�TH2O���Լ�Ϊ�ޢ�ڢ�٢ޣ���ѧ��ӦΪHNO3+NaOH�TNaNO3+H2O��H2SO4+2NaOH�T2H2O+Na2SO4��Ba��OH��2+2HNO3�T2H2O+Ba��NO3��2��
�ʴ�Ϊ���ޢߣ�HNO3+NaOH�TNaNO3+H2O��
���������ټ���Aʱ�����ɰ�ɫ����Ϊ̼�ᱵ����AΪBa��NO3��2������г�������Bʱ�������ܽ⣬��������ų�������Ϊ������̼������������ɵ���Һ�м���Cʱ�����а�ɫ�������ɣ��ð�ɫ����Ϊ���ᱵ����BΪHCl��CΪH2SO4���ʴ�Ϊ��Ba��NO3��2��HCl��H2SO4��
���������⿼�����ӷ�Ӧ���������ȡ��ע�⣨һ��ʵ���ҳ��ô���ʯ��CaCO3����ϡ�����ϡ���ᷴӦ��ȡCO2������Zn��ϡ�����ϡ���ᷴӦ��ȡ������
��������Na2CO3��Һ�м���A�����ɰ�ɫ��������AΪBa��NO3��2��������Ӧ��Na2CO3+Ba��NO3��2�TBaCO3��+2NaNO3������Bʱ�������ܽ⣬��BΪHCl��������Ӧ��BaCO3+2HCl�TBaCl2+H2O+CO2������CΪʣ���H2SO4����ȷ�����㼴�ɽ����Ŀ�ѶȲ���ע�ػ���֪ʶ�Ŀ��飮
��������Na2CO3��Һ�м���A�����ɰ�ɫ��������AΪBa��NO3��2��������Ӧ��Na2CO3+Ba��NO3��2�TBaCO3��+2NaNO3������Bʱ�������ܽ⣬��BΪHCl��������Ӧ��BaCO3+2HCl�TBaCl2+H2O+CO2������CΪʣ���H2SO4����ȷ�����㼴�ɽ����Ŀ�ѶȲ���ע�ػ���֪ʶ�Ŀ��飮

��ϰ��ϵ�д�
�����Ŀ
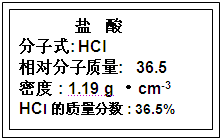
 �Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
�Լ�ƿ��ǩ�ϵ��й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
 _
_ __
__ ��Ӧ�����ӷ���ʽΪ____________________________________��
��Ӧ�����ӷ���ʽΪ____________________________________��