��Ŀ����
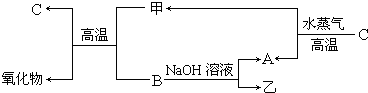
 A��B��C�����ֳ���������Ԫ�صĵ��ʣ�������DΪ��ɫҺ�壬E��һ�ֳ������������壮��ת����ϵ��ͼ����Ӧ�����Ͳ��ֲ�����ȥ�����Իش�
A��B��C�����ֳ���������Ԫ�صĵ��ʣ�������DΪ��ɫҺ�壬E��һ�ֳ������������壮��ת����ϵ��ͼ����Ӧ�����Ͳ��ֲ�����ȥ�����Իش���1��E�Ľṹʽ��
O=C=O
O=C=O
��F�ĵ���ʽ��

��2��д��F��D��Ӧ�����ӷ���ʽ���������ת�Ƶķ������Ŀ


��3������Z��B��Ϊͬ�������壬�������ڶԿ�������ɱ����������ˮ�����ʵȣ�Z�����Ե⻯����Һ��Ӧ����B�͵ⵥ�ʣ���Ӧ�����ӷ���ʽ��
O3+2H++2I-=O2+I2+H2O
O3+2H++2I-=O2+I2+H2O
����4��ȡ0.3mol F������D��ֻ�Ϻ���ͨ��0.4mol Eǡ����ȫ�����գ�������Һ�и������ӵ�Ũ���ɴ�С��˳����
c��Na+����c��HCO3-����c��CO32-����c��OH-����c��H+��
c��Na+����c��HCO3-����c��CO32-����c��OH-����c��H+��
��������������DΪ��ɫҺ�壬ӦΪH2O��E��һ�ֳ������������壬ӦΪCO2����AΪH2��BΪO2��CΪCԪ���γɵĵ��ʣ�FΪNa2O2��
��1��CO2Ϊֱ���η��ӣ�Na2O2Ϊ���ӻ����
��2��F��D������ӦΪ2Na2O2+2H2O=4Na++4OH-+O2����
��3��ZӦΪO3����KI��Ӧ����O2��I2��
��4��0.3mol������ˮ��Ӧ����NaOH0.6mol����0.4molCO2��Ӧ����NaHCO3��Na2CO3��
��1��CO2Ϊֱ���η��ӣ�Na2O2Ϊ���ӻ����
��2��F��D������ӦΪ2Na2O2+2H2O=4Na++4OH-+O2����
��3��ZӦΪO3����KI��Ӧ����O2��I2��
��4��0.3mol������ˮ��Ӧ����NaOH0.6mol����0.4molCO2��Ӧ����NaHCO3��Na2CO3��
����⣺������DΪ��ɫҺ�壬ӦΪH2O��E��һ�ֳ������������壬ӦΪCO2����AΪH2��BΪO2��CΪCԪ���γɵĵ��ʣ�FΪNa2O2����
��1��CO2Ϊֱ���η��ӣ��ṹʽΪO=C=O��Na2O2Ϊ���ӻ��������ʽΪ ��
��
�ʴ�Ϊ��O=C=O�� ��
��
��2��F��D������ӦΪ2Na2O2+2H2O=4Na++4OH-+O2������Ӧ��Na2O2��������������ԭ��Ӧ����ʧ���ӿɱ�ʾΪ ��
��
�ʴ�Ϊ�� ��
��
��3��ZӦΪO3����KI��Ӧ����O2��I2����Ӧ�����ӷ���ʽΪO3+2H++2I-=O2+I2+H2O��
�ʴ�Ϊ��O3+2H++2I-=O2+I2+H2O��
��4��0.3mol������ˮ��Ӧ����NaOH0.6mol����0.4molCO2��Ӧ����NaHCO3��Na2CO3��
����n��CO2����n��NaOH��=0.4mol��0.6mol=2��3���ɽ���ѧ����ʽΪдΪ��2CO2+3NaOH=NaHCO3+Na2CO3+H2O��
�ɷ���ʽ��֪��n��NaHCO3��=0.2mol��n��Na2CO3��=0.2mol��
����CO32-ˮ������HCO3-����c��HCO3-����c��CO32-������Һ�ʼ��ԣ���c��OH-����c��H+����
һ����˵�������ˮ��̶Ƚ�С������c��CO32-����c��OH-����
����c��Na+����c��HCO3-����c��CO32-����c��OH-����c��H+����
�ʴ�Ϊ��c��Na+����c��HCO3-����c��CO32-����c��OH-����c��H+����
��1��CO2Ϊֱ���η��ӣ��ṹʽΪO=C=O��Na2O2Ϊ���ӻ��������ʽΪ
 ��
���ʴ�Ϊ��O=C=O��
 ��
����2��F��D������ӦΪ2Na2O2+2H2O=4Na++4OH-+O2������Ӧ��Na2O2��������������ԭ��Ӧ����ʧ���ӿɱ�ʾΪ
 ��
���ʴ�Ϊ��
 ��
����3��ZӦΪO3����KI��Ӧ����O2��I2����Ӧ�����ӷ���ʽΪO3+2H++2I-=O2+I2+H2O��
�ʴ�Ϊ��O3+2H++2I-=O2+I2+H2O��
��4��0.3mol������ˮ��Ӧ����NaOH0.6mol����0.4molCO2��Ӧ����NaHCO3��Na2CO3��
����n��CO2����n��NaOH��=0.4mol��0.6mol=2��3���ɽ���ѧ����ʽΪдΪ��2CO2+3NaOH=NaHCO3+Na2CO3+H2O��
�ɷ���ʽ��֪��n��NaHCO3��=0.2mol��n��Na2CO3��=0.2mol��
����CO32-ˮ������HCO3-����c��HCO3-����c��CO32-������Һ�ʼ��ԣ���c��OH-����c��H+����
һ����˵�������ˮ��̶Ƚ�С������c��CO32-����c��OH-����
����c��Na+����c��HCO3-����c��CO32-����c��OH-����c��H+����
�ʴ�Ϊ��c��Na+����c��HCO3-����c��CO32-����c��OH-����c��H+����
���������⿼��������ƶϣ���Ŀ�ƶ��ѶȲ��������ۺ϶Ƚϸߣ�����ע���������Ũ�ȴ�С�ıȽϷ�����

��ϰ��ϵ�д�
�����Ŀ
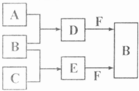
 �ס��ҡ������ֳ������ʣ�A��B��C�����ֳ����Ļ����AΪ����ɫ���壻����֮���ת����ϵ��ͼ��ʾ������д���пհף�
�ס��ҡ������ֳ������ʣ�A��B��C�����ֳ����Ļ����AΪ����ɫ���壻����֮���ת����ϵ��ͼ��ʾ������д���пհף�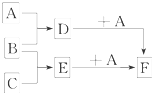 ��֪A��B��C�����ֳ����ĵ��ʣ�����AΪ���壬B��CΪ���壻D�ı�����Һ�����ˮ�м�����У���Һ�ʺ��ɫ��B��C��Ӧʱ�ɹ۲쵽��ɫ���棬����K������ˮ����ɫ��ҺE������֮��ת����ϵ��ͼ��ʾ��
��֪A��B��C�����ֳ����ĵ��ʣ�����AΪ���壬B��CΪ���壻D�ı�����Һ�����ˮ�м�����У���Һ�ʺ��ɫ��B��C��Ӧʱ�ɹ۲쵽��ɫ���棬����K������ˮ����ɫ��ҺE������֮��ת����ϵ��ͼ��ʾ��