��Ŀ����
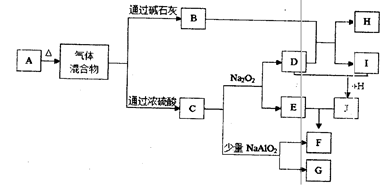
��ѧ��ѧ���ֳ������ʵ�ת����ϵ���£���Щ����������ȥ���� ����A�dz����Ľ������ʣ�F�����ǹ�ҵ����ȡ�ռ��ԭ�ϣ�G��H�dz����ķǽ������ʣ�����Ϊ�������ش��������⣺
��1��д��ָ�����ʵĻ�ѧʽ��A ��C ��H ��
��2��д�����з�Ӧ�����ӷ���ʽ��
��D+I��E+F ��
��C������ʵ�����Na2O2����Һ�з�Ӧ�� ��
��3����D�ı�����Һ�����Ƶ�E���壬�ý�����E����ֱ���Ĵ�С��Χ�� ����Ҫ�ᴿE���壬���õIJ��������� ��
��4��D��ˮ��Һ�� �ԣ������ԡ����Ի����ԣ���Ϊ������һ�仯��ͨ��������D��Һʱ�� ��ȡ�ķ����� ��
��ȡ�ķ����� ��

��1��д��ָ�����ʵĻ�ѧʽ��A ��C ��H ��
��2��д�����з�Ӧ�����ӷ���ʽ��
��D+I��E+F ��
��C������ʵ�����Na2O2����Һ�з�Ӧ�� ��
��3����D�ı�����Һ�����Ƶ�E���壬�ý�����E����ֱ���Ĵ�С��Χ�� ����Ҫ�ᴿE���壬���õIJ��������� ��
��4��D��ˮ��Һ�� �ԣ������ԡ����Ի����ԣ���Ϊ������һ�仯��ͨ��������D��Һʱ��
 ��ȡ�ķ����� ��
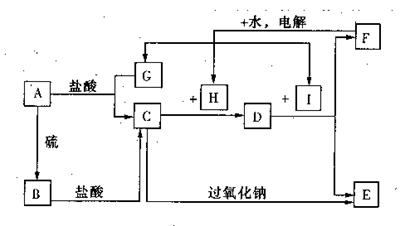
��ȡ�ķ����� ����1��Fe FeCl2 Cl2����1�֣�
��2����Fe3++3OH- Fe(OH)3����2�֣�
��4Fe2++4Na2O2+6H2O 4Fe(OH)3��+O2��+8Na+��2�֣�
��3��1nm~100nm��2�֣� ������2�֣�
��4���ᣨ1�֣� ��FeCl3���ڽ�Ũ�������У�����ˮϡ�͵������Ũ�ȣ�2�֣�
��2����Fe3++3OH-
��4Fe2++4Na2O2+6H2O
��3��1nm~100nm��2�֣� ������2�֣�
��4���ᣨ1�֣� ��FeCl3���ڽ�Ũ�������У�����ˮϡ�͵������Ũ�ȣ�2�֣�
��

��ϰ��ϵ�д�
�����Ŀ
 ��Ԫ������������ˮ�����������ǿ���� ���ѧʽ�������л�ѧ�������Բ�ͬ���������ֻ������
��Ԫ������������ˮ�����������ǿ���� ���ѧʽ�������л�ѧ�������Բ�ͬ���������ֻ������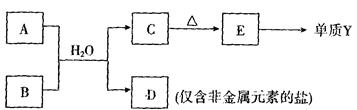
 2CA3��g��
2CA3��g�� .0 L��
.0 L��